Label: FEXOFENADINE HYDROCHLORIDE tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 63940-769-03, 63940-769-70 - Packager: HARMON STORES INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 16, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Do not use
- Ask a doctor before use if you have
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions
- Other Information
- Inactive ingredients
- Questions?
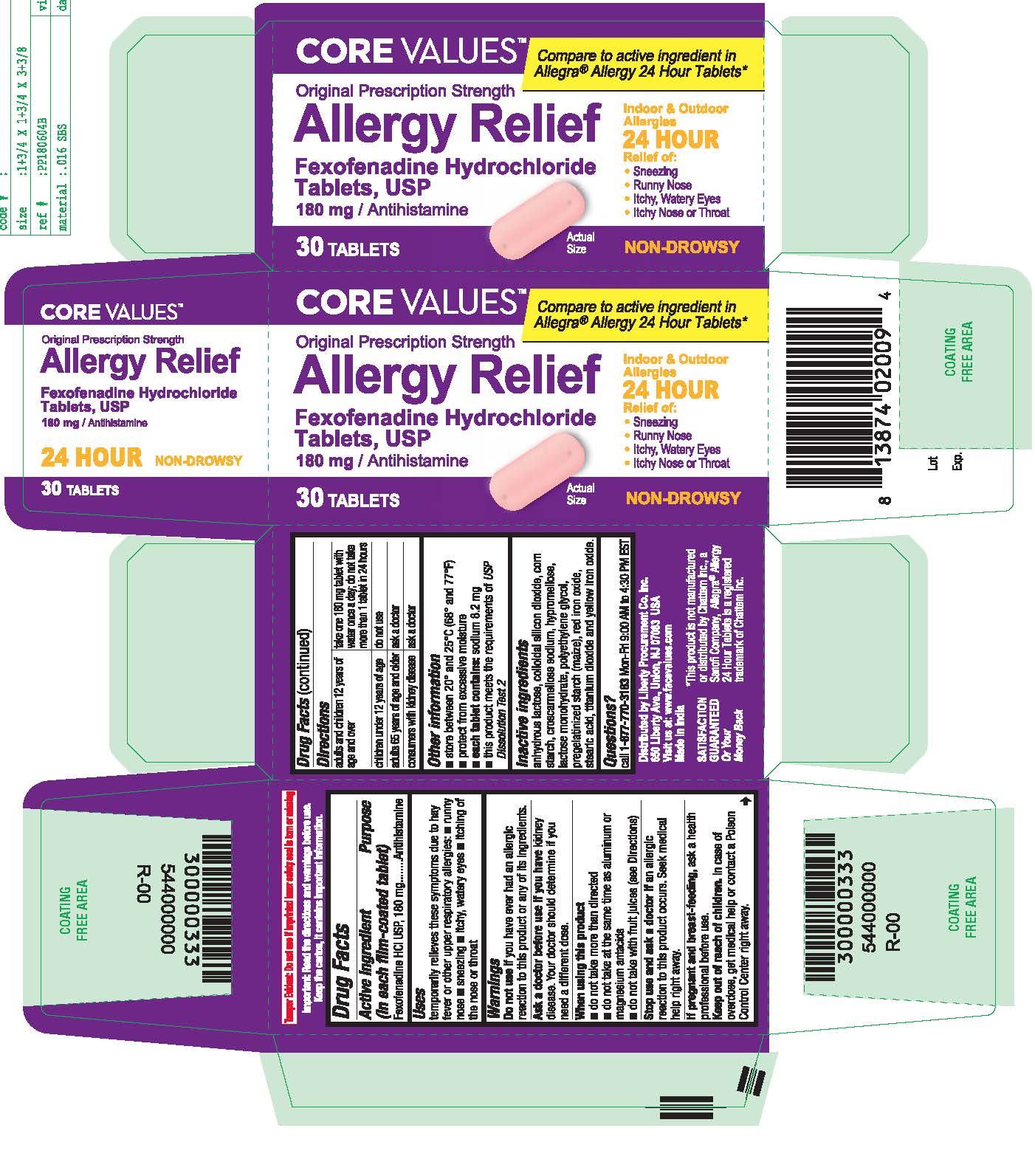
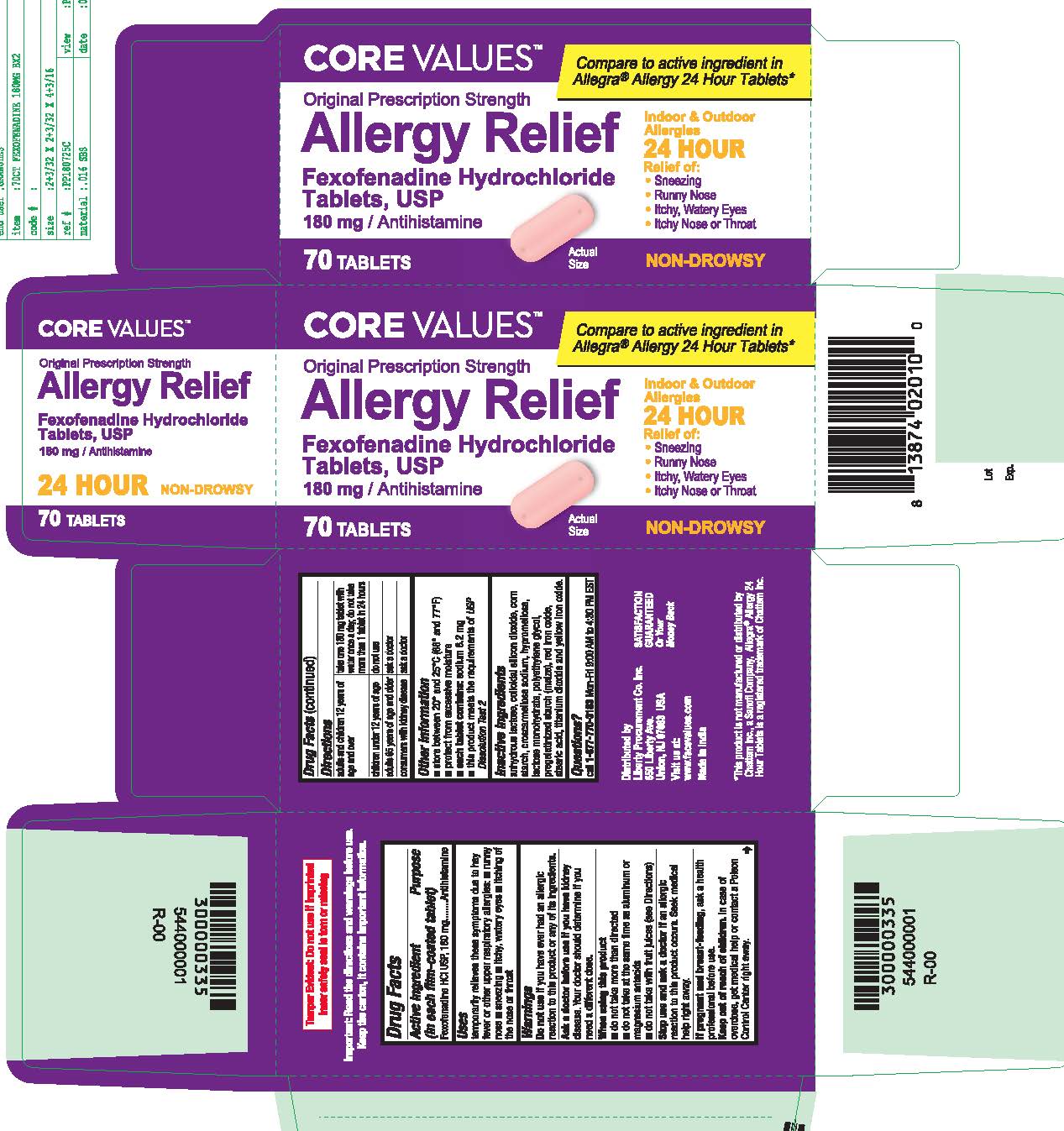
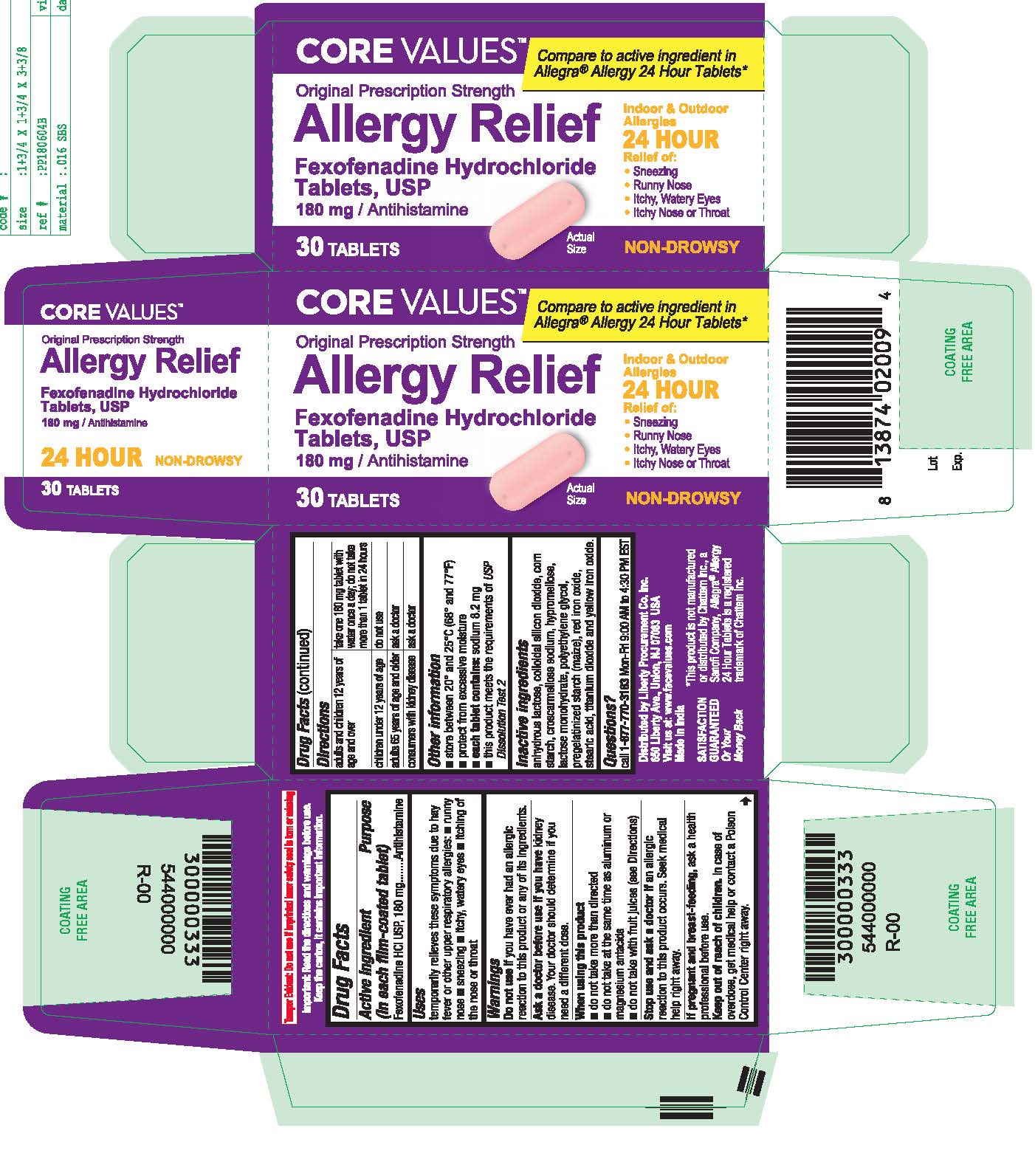
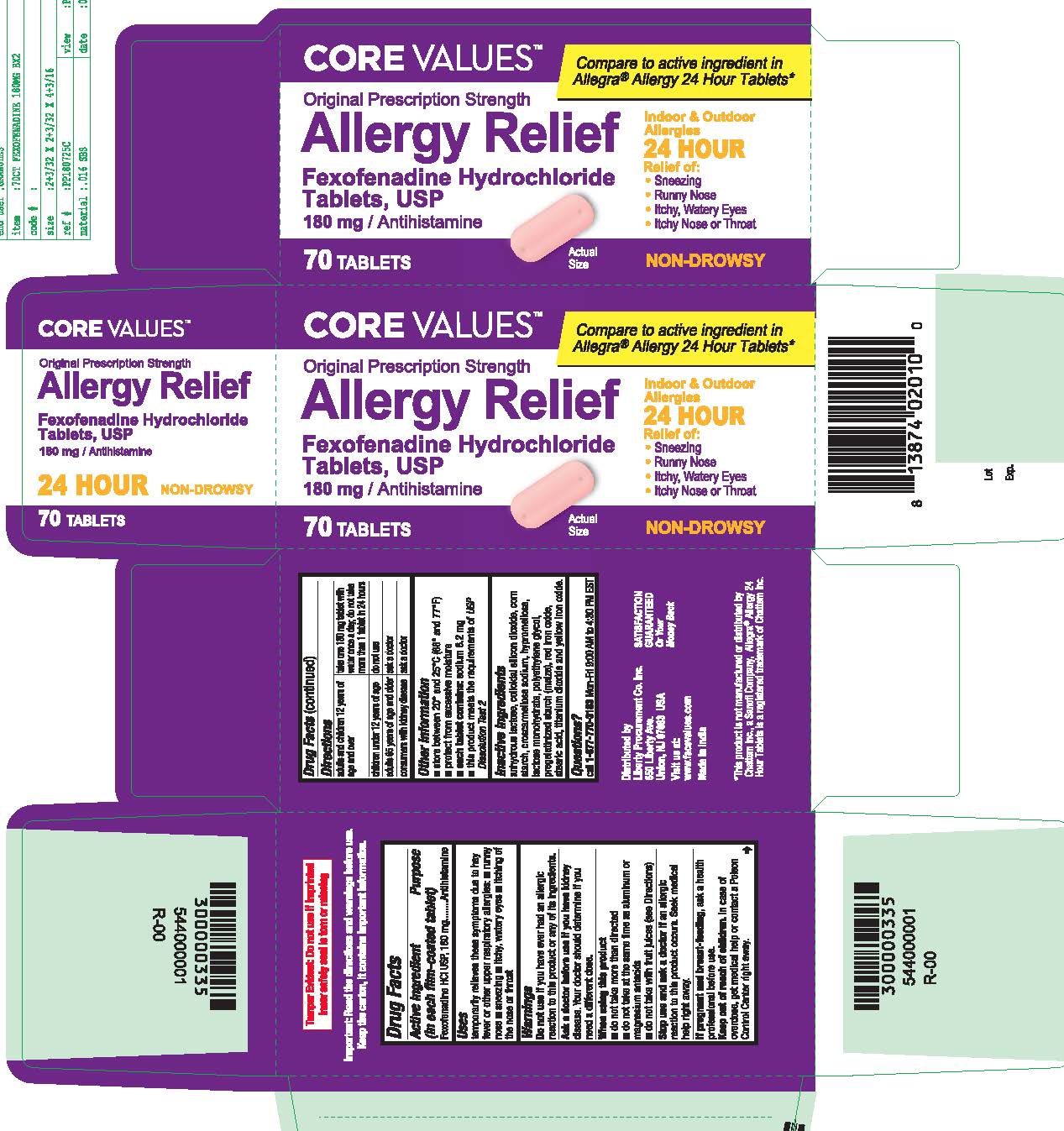
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FEXOFENADINE HYDROCHLORIDE
fexofenadine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63940-769 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) FERRIC OXIDE RED (UNII: 1K09F3G675) STEARIC ACID (UNII: 4ELV7Z65AP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color pink Score no score Shape OVAL (Capsule Shaped Tablets) Size 17mm Flavor Imprint Code SG;202 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63940-769-03 30 in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2018 2 NDC:63940-769-70 70 in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204507 10/01/2018 Labeler - HARMON STORES INC. (804085293)