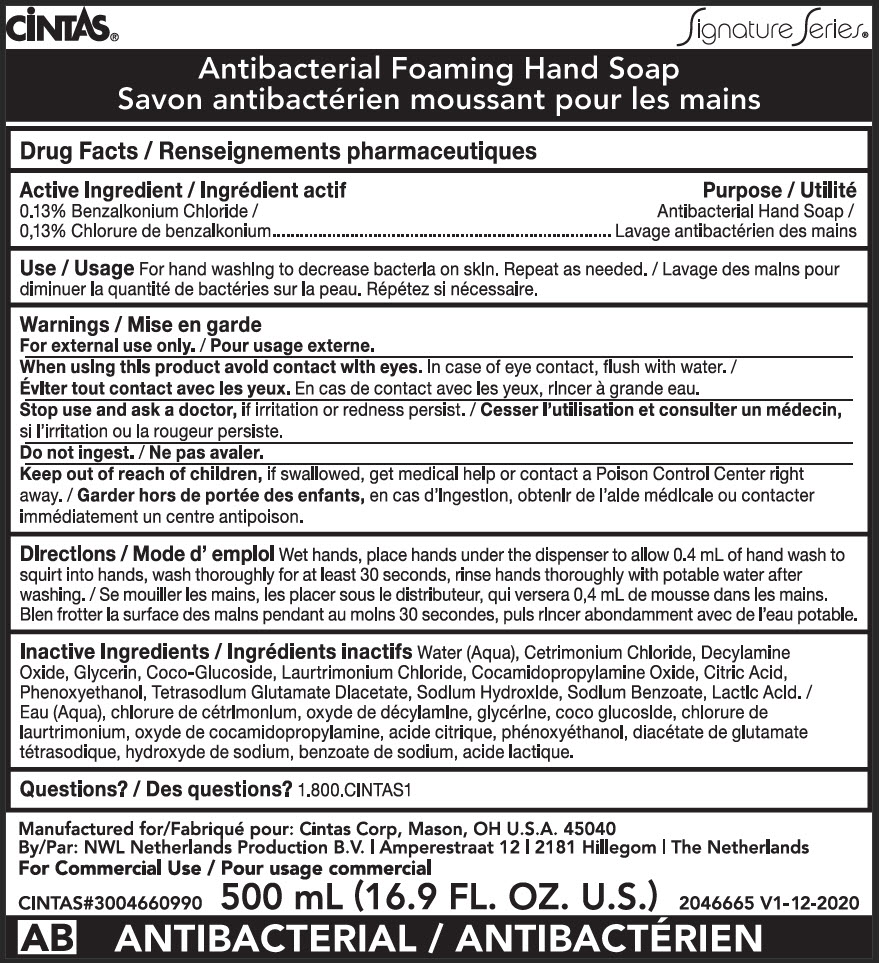
Label: ANTIBACTERIAL FOAMING HAND- benzalkonium chloride soap
- NDC Code(s): 42961-615-01
- Packager: Cintas
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Use
-
Warnings
Keep out of reach of children, if swallowed, get medical help or contact a Poison Control Center right away.
Directions
Wet hands, place hands under the dispenser to allow 0.4 mL of hand wash to squirt into hands, wash thoroughly for at least 30 seconds, rinse hands thoroughly with potable water after washing.
- PRINCIPAL DISPLAY PANEL - 500 mL Bag Label
-
INGREDIENTS AND APPEARANCE
ANTIBACTERIAL FOAMING HAND
benzalkonium chloride soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42961-615 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 13 mg in 10 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) DECYLAMINE OXIDE (UNII: G387VUT5EZ) GLYCERIN (UNII: PDC6A3C0OX) COCO GLUCOSIDE (UNII: ICS790225B) LAURTRIMONIUM CHLORIDE (UNII: A81MSI0FIC) COCAMIDOPROPYLAMINE OXIDE (UNII: M4SL82J7HK) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PHENOXYETHANOL (UNII: HIE492ZZ3T) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM BENZOATE (UNII: OJ245FE5EU) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42961-615-01 10 in 1 CARTON 06/01/2022 1 500 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH DRUG M003 06/01/2022 Labeler - Cintas (056481716) Establishment Name Address ID/FEI Business Operations NWL Netherlands Services B.V. 494692088 ANALYSIS(42961-615) , MANUFACTURE(42961-615)