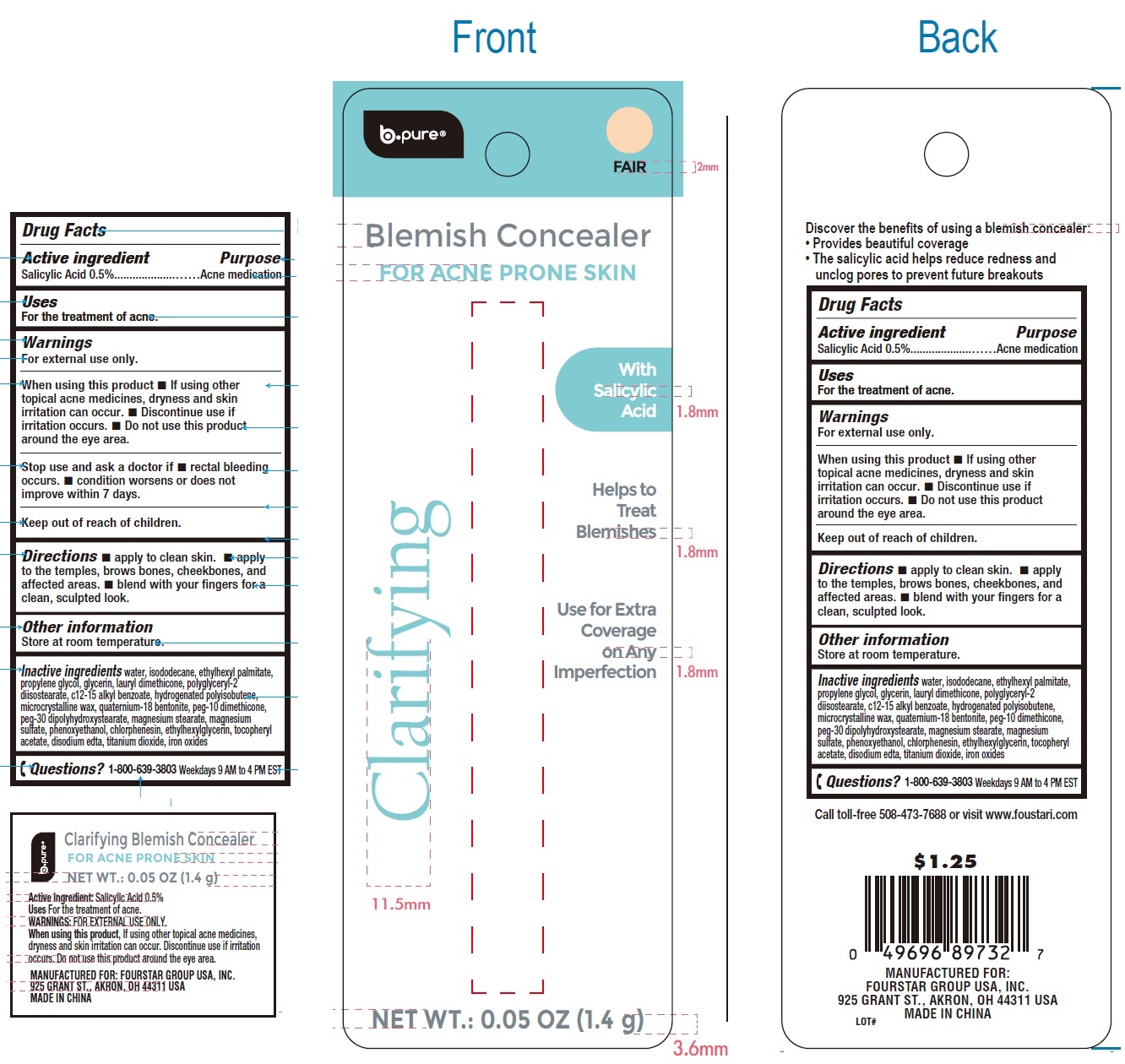
Label: B PURE BLEMISH CONCEALER FOR ACNE PRONE SKIN FAIR- salicylic acid cream
- NDC Code(s): 80684-109-00
- Packager: Fourstar Group USA, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
-
Warnings
For external use only.
When using this product
- If using other topical acne medicines, dryness and skin irritation can occur.
- Discontinue use if irritation occurs.
- Do not use this product around the eye area.
- Directions
- Other information
-
Inactive ingredients
water, isododecane, ethylhexyl palmitate, propylene glycol, glycerin, lauryl dimethicone, polyglyceryl-2 diisostearate, c12-15 alkyl benzoate, hydrogenated polyisobutene, microcrystalline wax, quaternium-18 bentonite, peg-10 dimethicone, peg-30 dipolyhydroxystearate, magnesium stearate, magnesium sulfate, phenoxyethanol, chlorphenesin, ethylhexylglycerin, tocopheryl acetate, disodium edta, titanium dioxide, iron oxides
- Questions?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
B PURE BLEMISH CONCEALER FOR ACNE PRONE SKIN FAIR
salicylic acid creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80684-109 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISODODECANE (UNII: A8289P68Y2) ETHYLHEXYL PALMITATE (UNII: 2865993309) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) POLYGLYCERYL-2 DIISOSTEARATE (UNII: DG195GP57P) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) BENTOQUATAM (UNII: 7F465U79Q1) PEG-30 DIPOLYHYDROXYSTEARATE (4000 MW) (UNII: 9713Q0S7FO) MAGNESIUM STEARATE (UNII: 70097M6I30) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) EDETATE DISODIUM (UNII: 7FLD91C86K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80684-109-00 1.4 g in 1 TUBE; Type 0: Not a Combination Product 09/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 09/01/2024 Labeler - Fourstar Group USA, Inc. (140099503)