Label: EUCERIN DAILY PROTECTION FACE- ensulizole, octinoxate, octisalate, titanium dioxide, zinc oxide lotion
- NDC Code(s): 10356-323-04, 10356-323-11, 10356-323-25, 10356-323-27
- Packager: Beiersdorf Inc
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 29, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
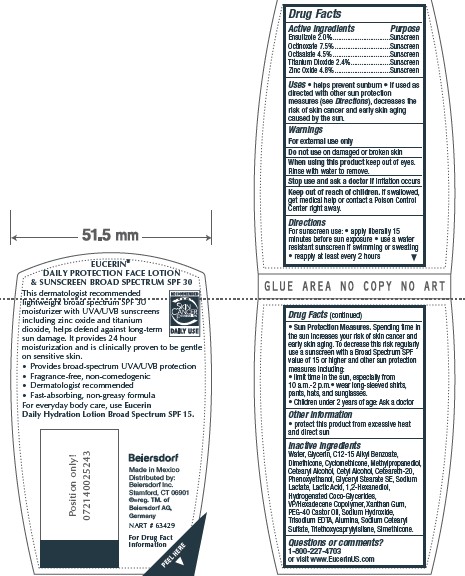
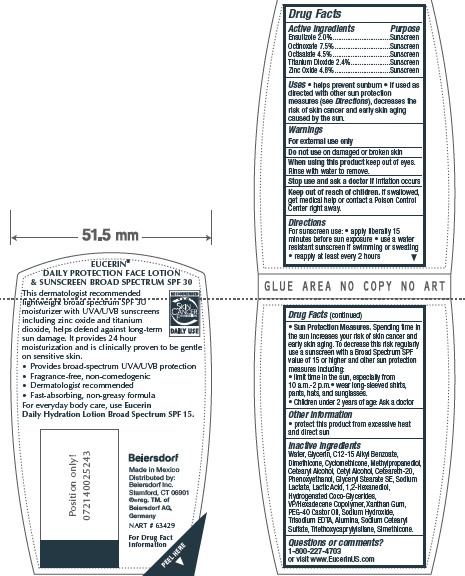
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
For sunscreen use: • apply liberally 15
minutes before sun exposure • use a water
resistant sunscreen if swimming or sweating
• reapply at least every 2 hours
• Sun Protection Measures. Spending time in
the sun increases your risk of skin cancer and
early skin aging. To decrease this risk regularly
use a sunscreen with a Broad Spectrum SPF
value of 15 or higher and other sun protection
measures including:
• limit time in the sun, especially from
10 a.m.-2 p.m.• wear long-sleeved shirts,
pants, hats, and sunglasses.
• Children under 6 months of age: Ask a doctor -
INACTIVE INGREDIENT
Inactive Ingredients
Water, Glycerin, C12-15 Alkyl Benzoate,
Dimethicone, Cyclomethicone, Methylpropanediol,
Cetearyl Alcohol, Cetyl Alcohol, Ceteareth-20,
Phenoxyethanol, Glyceryl Stearate SE, Sodium
Lactate, Lactic Acid, 1,2-Hexanediol,
Hydrogenated Coco-Glycerides,
VP/Hexadecene Copolymer, Xanthan Gum,
PEG-40 Castor Oil, Sodium Hydroxide,
Trisodium EDTA, Alumina, Sodium Cetearyl
Sulfate, Triethoxycaprylylsilane, Simethicone
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EUCERIN DAILY PROTECTION FACE
ensulizole, octinoxate, octisalate, titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10356-323 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ENSULIZOLE (UNII: 9YQ9DI1W42) (ENSULIZOLE - UNII:9YQ9DI1W42) ENSULIZOLE 2 g in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.5 g in 100 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 2.4 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 4.8 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) DIMETHICONE (UNII: 92RU3N3Y1O) CYCLOMETHICONE (UNII: NMQ347994Z) CETYL ALCOHOL (UNII: 936JST6JCN) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) SODIUM LACTATE (UNII: TU7HW0W0QT) LACTIC ACID (UNII: 33X04XA5AT) COCO-GLYCERIDES (UNII: ISE9I7DNUG) POLYOXYL 40 CASTOR OIL (UNII: 4ERD2076EF) SODIUM CETOSTEARYL SULFATE (UNII: 7ZBS06BH4B) XANTHAN GUM (UNII: TTV12P4NEE) EDETATE TRISODIUM (UNII: 420IP921MB) SODIUM HYDROXIDE (UNII: 55X04QC32I) ALUMINUM OXIDE (UNII: LMI26O6933) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPROPANEDIOL (UNII: N8F53B3R4R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) VINYLPYRROLIDONE/HEXADECENE COPOLYMER (UNII: KFR5QEN0N9) TRIMETHOXYCAPRYLYLSILANE (UNII: FZ07E4LW2M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10356-323-04 30 mL in 1 TUBE; Type 0: Not a Combination Product 08/12/2015 2 NDC:10356-323-25 5 mL in 1 TUBE; Type 0: Not a Combination Product 08/12/2015 3 NDC:10356-323-27 4 mL in 1 PACKET; Type 0: Not a Combination Product 08/12/2019 4 NDC:10356-323-11 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/12/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/12/2015 Labeler - Beiersdorf Inc (001177906)