Label: CURODONT REPAIR FLUORIDE PLUS- sodium fluoride sponge
-
Contains inactivated NDC Code(s)
NDC Code(s): 72247-101-11, 72247-101-12 - Packager: CREDENTIS AG
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 11, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
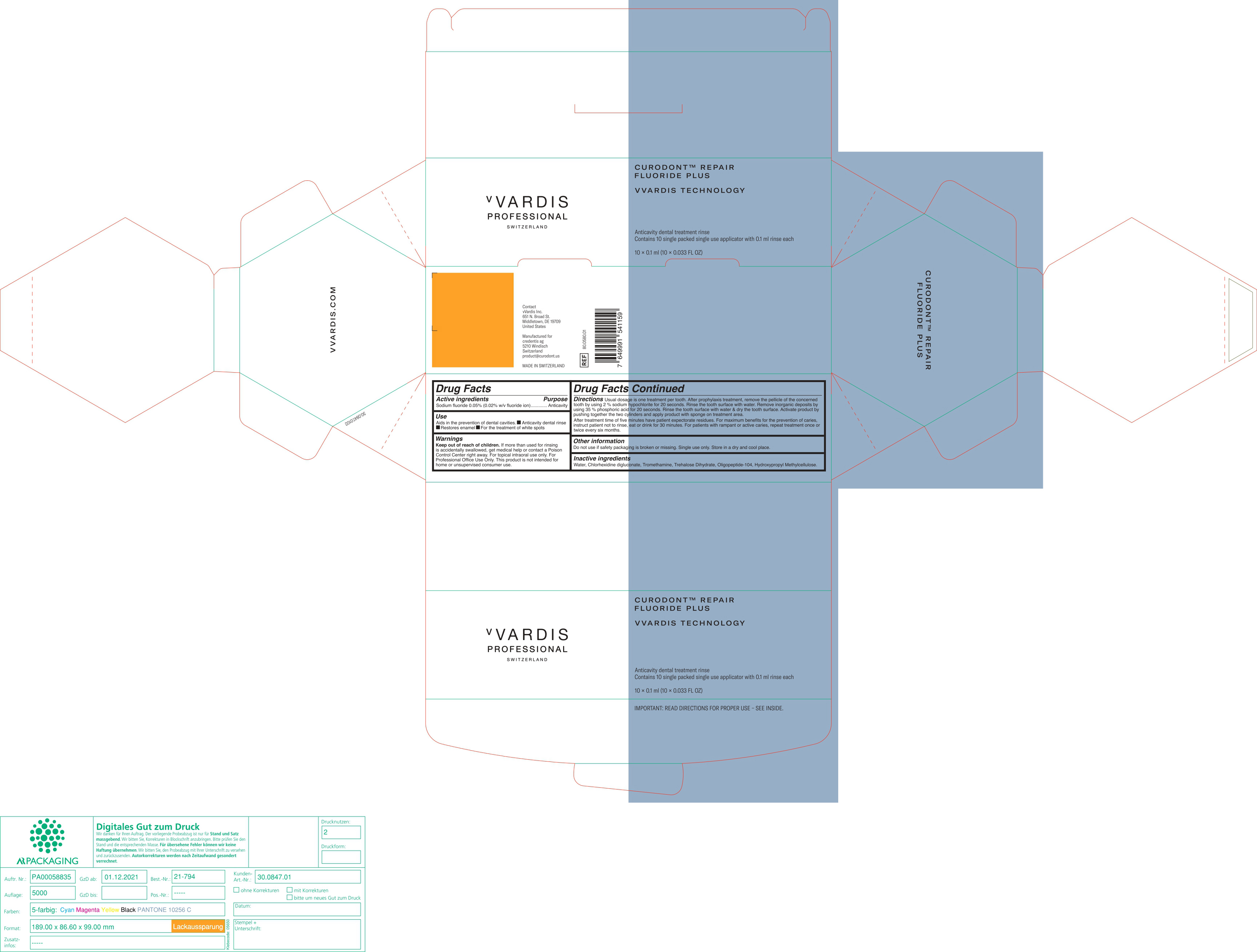
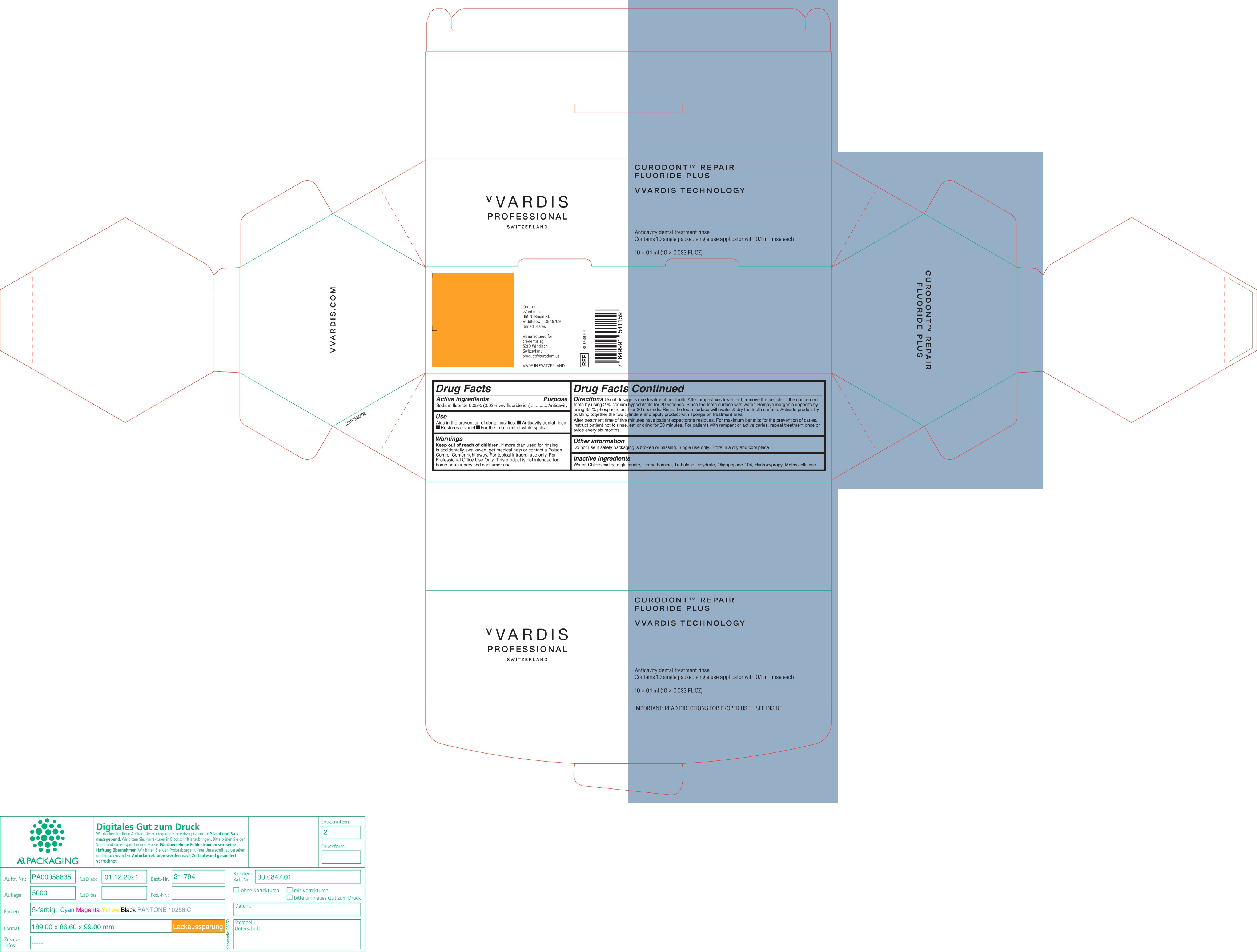
- ACTIVE INGREDIENTS
- PURPOSE
- USES
- WARNING
- KEEP OUT OF REACH OF CHILDREN
-
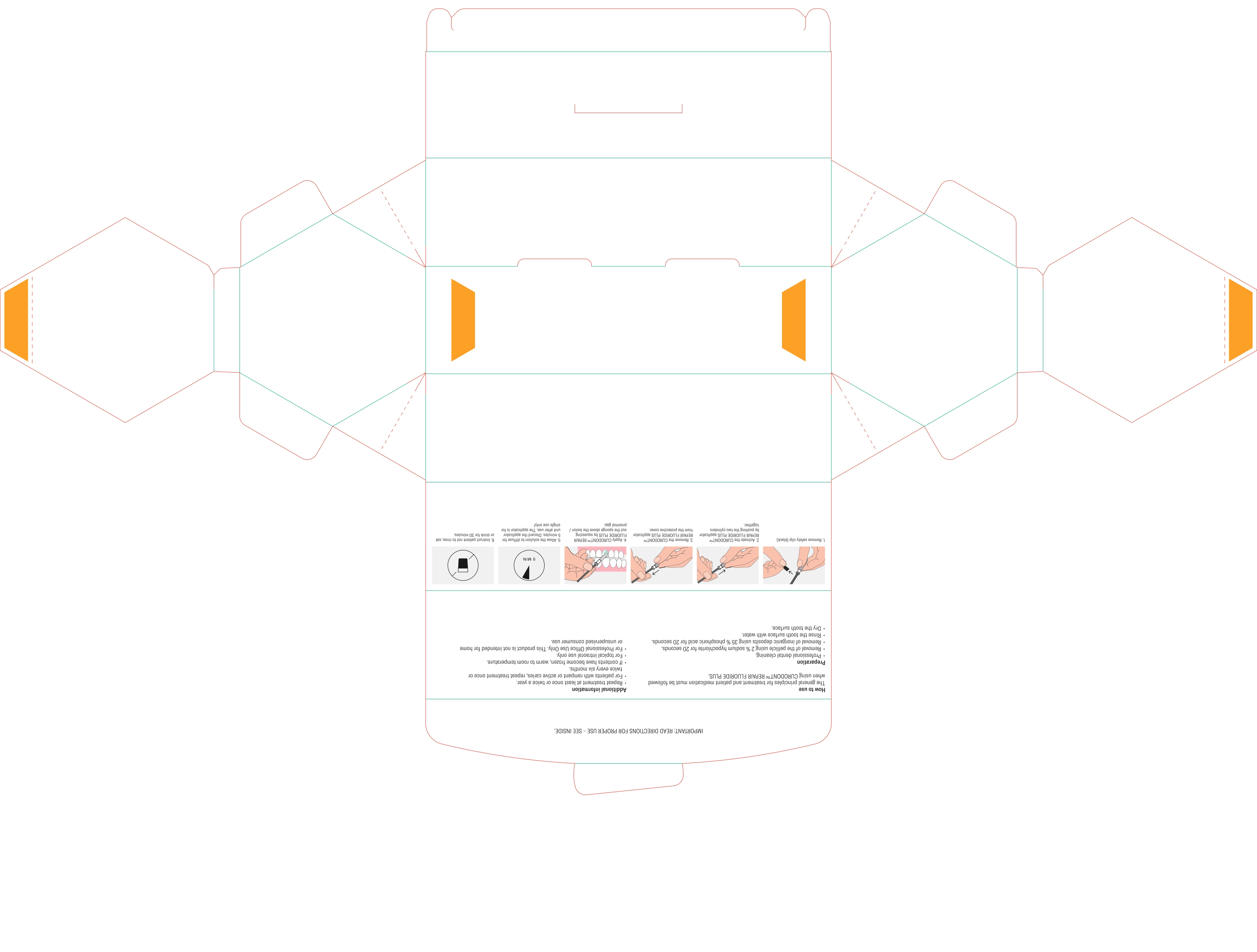
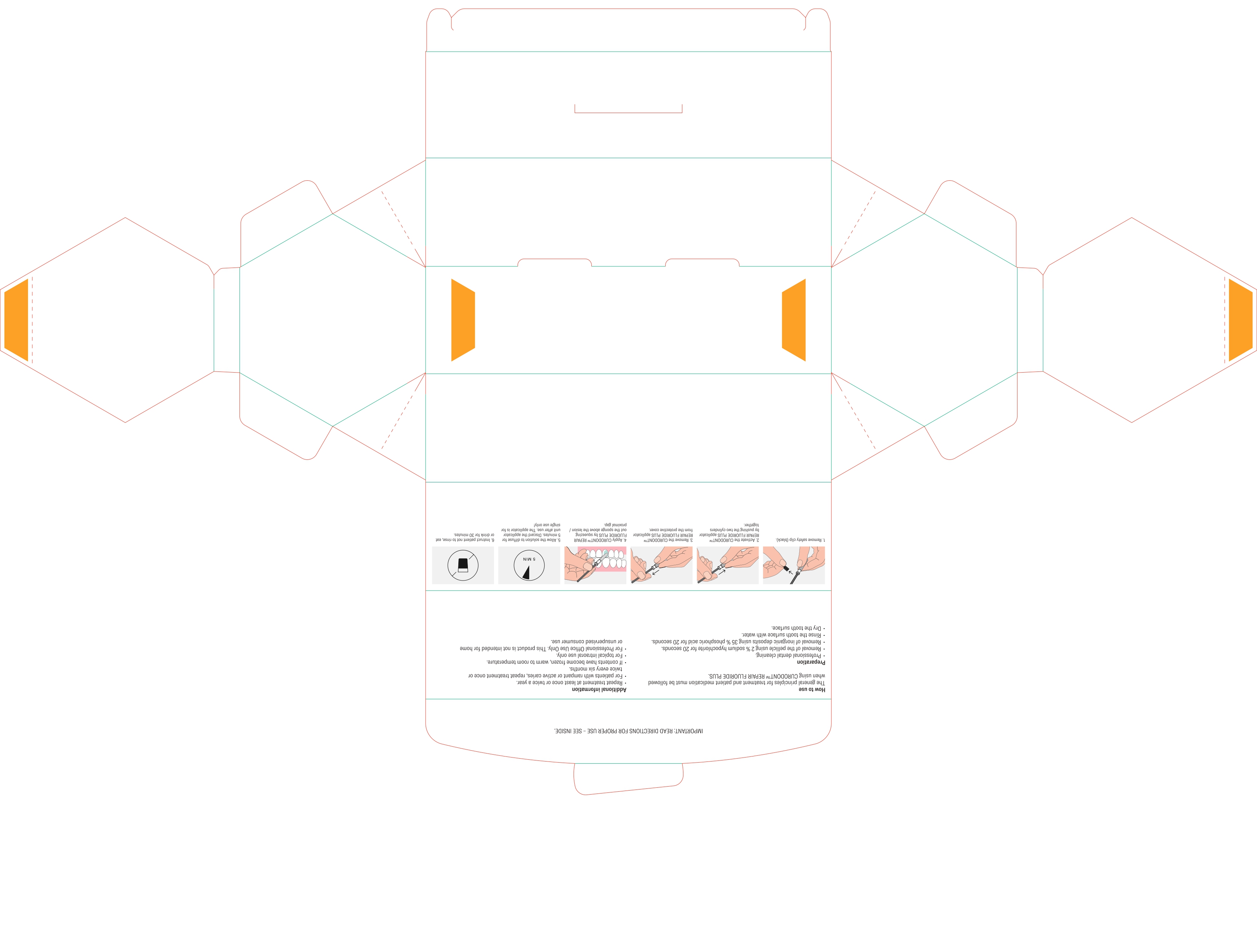
DIRECTIONS
Usual dosage is one treatment per tooth. After prophylaxis treatment, remove the pellicle of the concerned tooth by using 2% sodium hypochlorite for 10 seconds. rinse the tooth surface with water. Remove inorganic deposits by pushing together the two cylinders and apply product with sponge on treatment area.
After treatment time of five minutes have patient expectorate residues. For maximum benefits for the prevention of caries, instruct patient not to rinse, eat or drink for 30 minutes. For patients with rampant or active caries, repeat treatment once or twice every six months.
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CURODONT REPAIR FLUORIDE PLUS
sodium fluoride spongeProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72247-101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM MONOFLUOROPHOSPHATE (UNII: C810JCZ56Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.02 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) TROMETHAMINE (UNII: 023C2WHX2V) TREHALOSE DIHYDRATE (UNII: 7YIN7J07X4) OLIGOPEPTIDE-10 (UNII: Q46328TRNK) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72247-101-12 10 in 1 BOX 01/11/2019 1 NDC:72247-101-11 0.1 mL in 1 CYLINDER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 01/11/2019 Labeler - CREDENTIS AG (485450345)