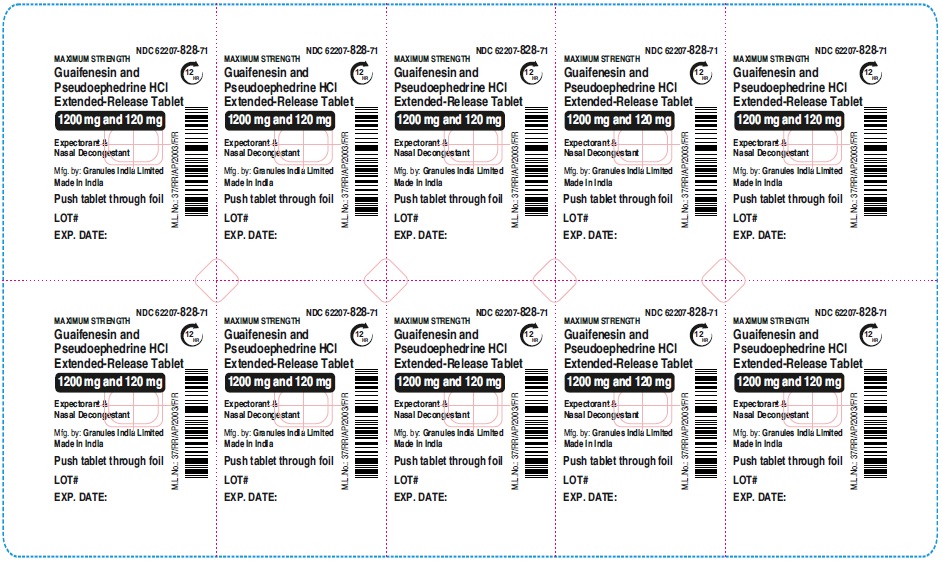
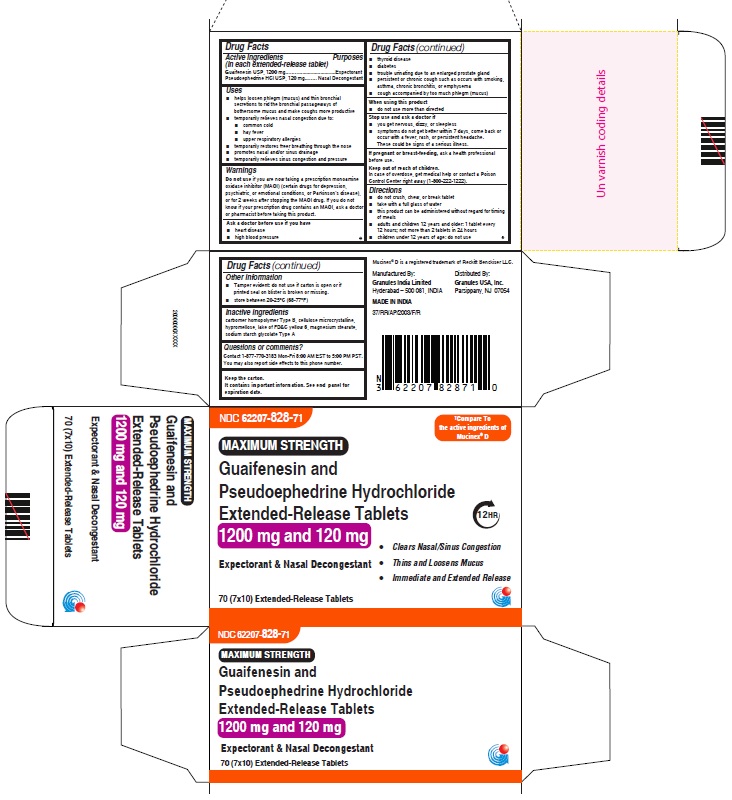
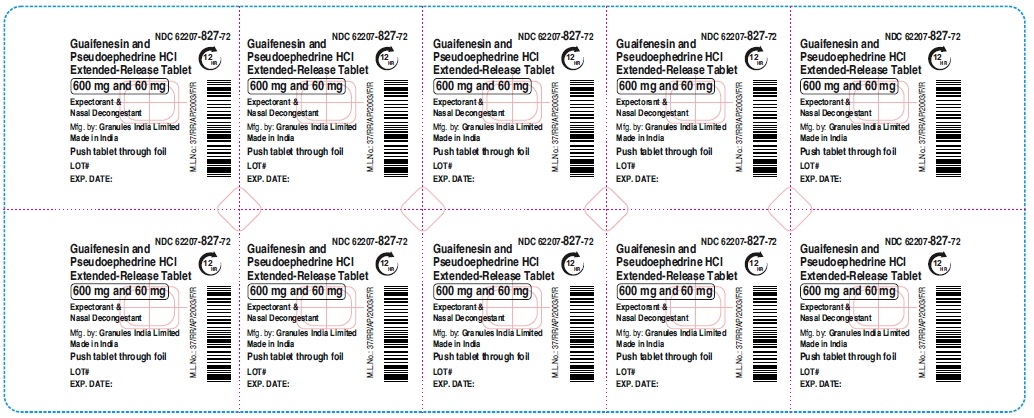
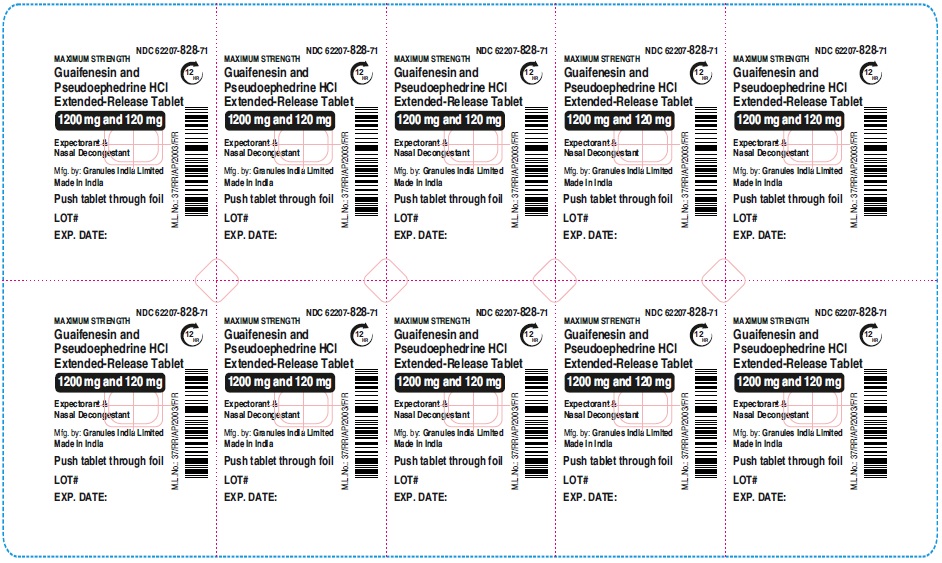
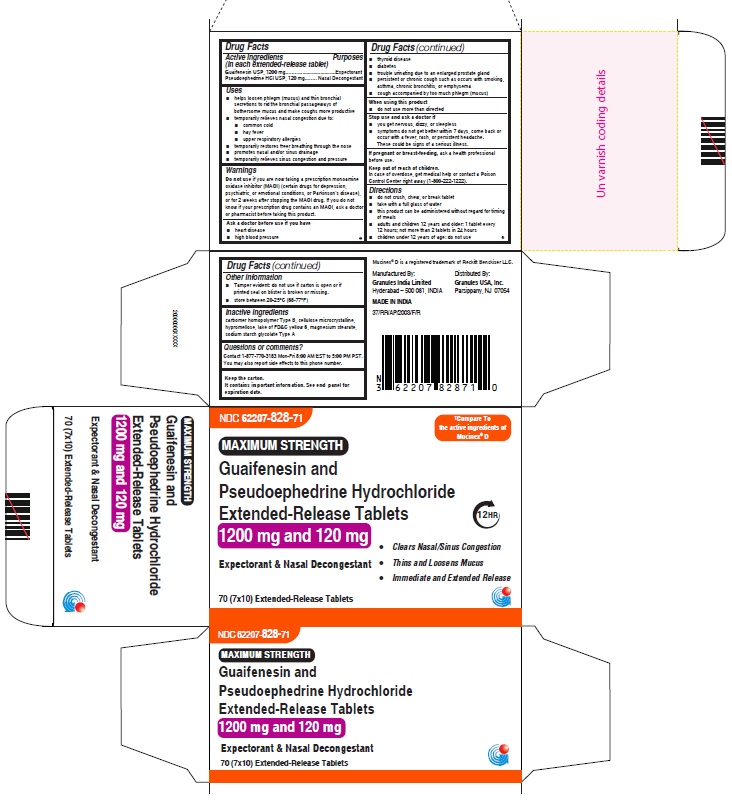
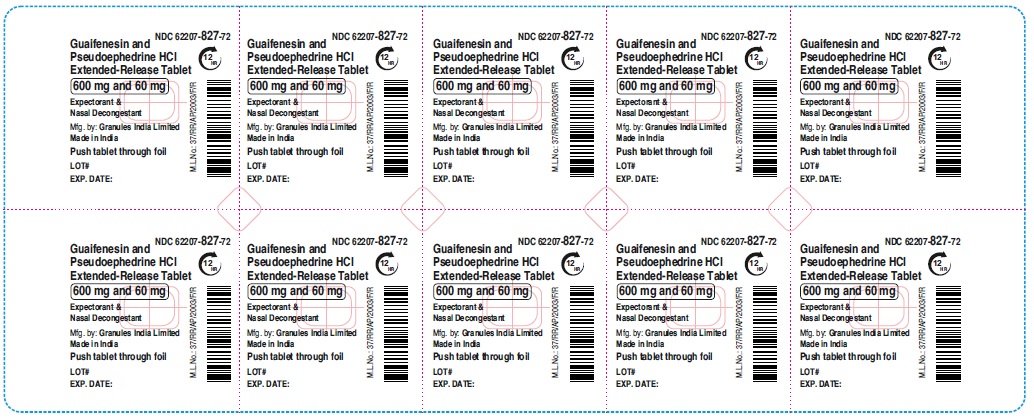
Label: GUAIFENESIN AND PSEUDOEPHEDRINE HCL tablet, extended release
- NDC Code(s): 62207-827-72, 62207-828-71
- Packager: Granules India Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENS
- PURPOSES
-
USES
• helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
• temporarily relieves nasal congestion due to:
• common cold
• hay fever
• upper respiratory allergies
• temporarily restores freer breathing through the nose
• promotes nasal and/or sinus drainage
• temporarily relieves sinus congestion and pressure -
WARNINGS
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- ASK A DOCTOR BEFORE USE IF YOU HAVE
- WHEN USING THIS PRODUCT
- STOP USE AND ASK A DOCTOR IF
- IF PREGNANT OR BREAST-FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
• do not crush, chew, or break tablet
• take with a full glass of water
• this product can be administered without regard for timing of meals
• adults and children 12 years and older:
For 600mg and 60mg: 2 tablets every 12 hours; not more than 4 tablets in 24 hours
For 1200mg and 120mg: 1 tablet every 12 hours; not more than 2 tablets in 24 hours
• children under 12 years of age: do not use - OTHER INFORMATION
- INACTIVE INGREDIENTS
-
QUESTIONS OR COMMENTS
Contact 1-877-770-3183 Mon-Fri 8:00 AM EST to 5:00 PM PST.
You may also report side effects to this phone number.
Keep the carton.
It contains important information. See end panel for
expiration date.
Manufactured By:
Granules India Limited
Hyderabad – 500 081, INDIA
MADE IN INDIADistributed By:
Granules USA, Inc.
Parsippany, NJ 07054
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN AND PSEUDOEPHEDRINE HCL
guaifenesin and pseudoephedrine hcl tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62207-828 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 1200 mg PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 120 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) HYPROMELLOSE 2910 (10000 MPA.S) (UNII: 0HO1H52958) Product Characteristics Color orange (Light orange and orange colored) Score no score Shape OVAL Size 22mm Flavor Imprint Code GP;1200 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62207-828-71 7 in 1 CARTON 06/01/2023 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216082 06/01/2023 GUAIFENESIN AND PSEUDOEPHEDRINE HCL
guaifenesin and pseudoephedrine hcl tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62207-827 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 600 mg PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 60 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) HYPROMELLOSE 2910 (10000 MPA.S) (UNII: 0HO1H52958) Product Characteristics Color orange (Light orange and orange colored) Score no score Shape OVAL Size 16mm Flavor Imprint Code GP;600 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62207-827-72 9 in 1 CARTON 06/01/2023 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216082 06/01/2023 Labeler - Granules India Ltd (915000087)