Label: CALCIUM CHLORIDE injection, solution
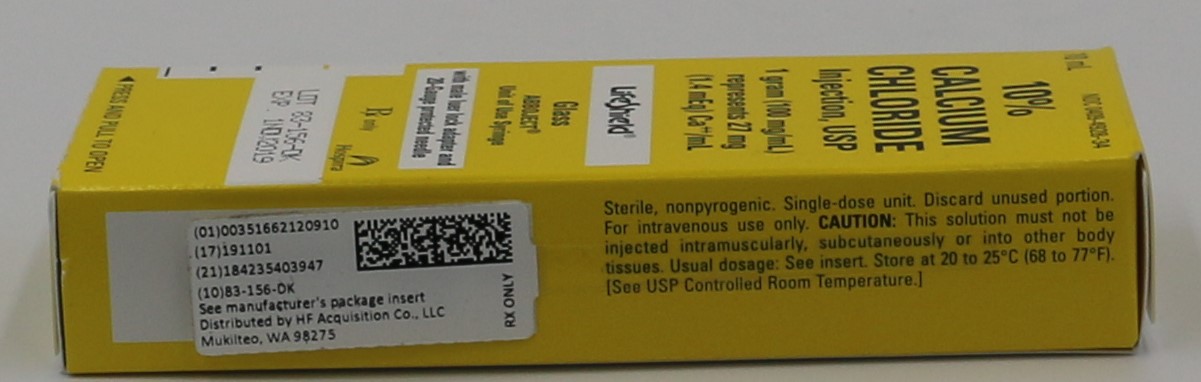
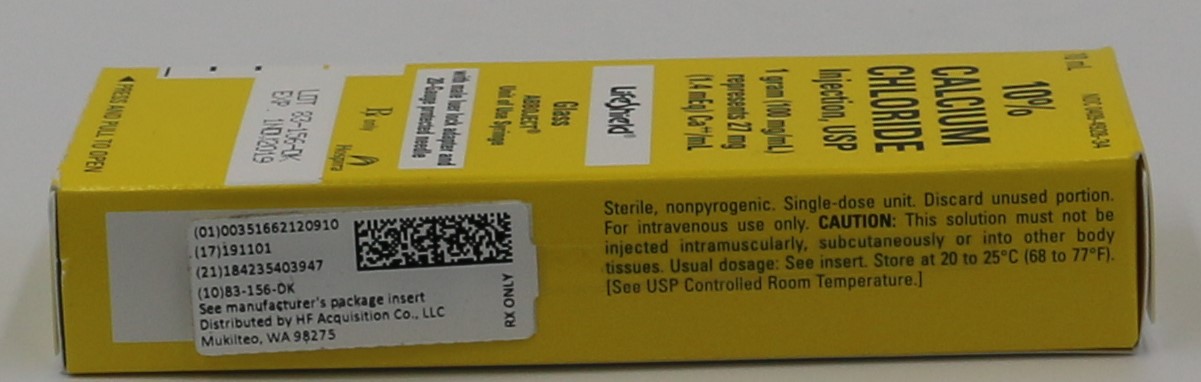
- NDC Code(s): 51662-1209-1, 51662-1209-2, 51662-1209-3
- Packager: HF Acquisition Co LLC, DBA HealthFirst
- This is a repackaged label.
- Source NDC Code(s): 0409-4928
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED
-
DESCRIPTION
10% Calcium Chloride Injection, USP is a sterile, nonpyrogenic, hypertonic solution containing 100 mg (1.4 mEq/mL) of calcium chloride, dihydrate (1.4 mEq each of Ca++ and Cl¯) in water for injection. It is provided in a 10 mL Unit of Use Syringe to facilitate prompt intravenous injection. The solution is administered only by intravenous or intraventricular cavity injection as a calcium replenisher.
The solution contains no bacteriostat, antimicrobial agent or added buffer and is intended only for use as a single-dose injection. As per USP testing, when diluted with water for injection to make a 5% solution, the pH of calcium chloride injection is 6.3 (5.5 to 7.5). May contain hydrochloric acid and/or sodium hydroxide for pH adjustment. The osmolar concentration is 2.04 mOsmol/mL (calc.). 10% Calcium Chloride Injection, USP is oxygen sensitive.
Calcium Chloride, USP dihydrate is chemically designated CaCl2 · 2H2O (dihydrate) white, odorless fragments or granules freely soluble in water.
-
CLINICAL PHARMACOLOGY
Calcium is the fifth most abundant element in the body and the major fraction is in the bony structure. Calcium plays important physiological roles, many of which are poorly understood. It is essential for the functional integrity of the nervous and muscular systems. It is necessary for normal cardiac function and is one of the factors that operates in the mechanisms involved in the coagulation of blood.
Calcium chloride in water dissociates to provide calcium (Ca++) and chloride (Cl¯) ions. They are normal constituents of the body fluids and are dependent on various physiological mechanisms for maintenance of balance between intake and output. Approximately 80% of body calcium is excreted in the feces as insoluble salts; urinary excretion accounts for the remaining 20%.
-
INDICATIONS & uSAGE
10% Calcium Chloride Injection, USP is indicated (1) for the treatment of hypocalcemia in those conditions requiring a prompt increase in blood plasma calcium levels, (2) in the treatment of magnesium intoxication due to overdosage of magnesium sulfate and (3) to combat the deleterious effects of hyperkalemia as measured by electrocardiographic (ECG), pending correction of the increased potassium level in the extracellular fluid.
10% Calcium Chloride Injection, USP also may be used in cardiac resuscitation when weak or inadequate contractions return following defibrillation or when epinephrine injection has failed to strengthen myocardial contractions.
- CONTRAINDICATIONS
-
WARNINGS
10% Calcium Chloride Injection, USP is irritating to veins and must not be injected into tissues, since severe necrosis and sloughing may occur. Great care should be taken to avoid extravasation or accidental injection into perivascular tissues.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
-
PRECAUTIONS
Do not administer unless solution is clear and seal is intact. Discard unused portion.
Because of its additive effect, calcium should be administered very cautiously to a patient who is digitalized or who is taking effective doses of digitalis or digitalis-like preparations.
Injections should be made slowly through a small needle into a large vein to minimize venous irritation and avoid undesirable reactions. It is particularly important to prevent a high concentration of calcium from reaching the heart because of the danger of cardiac syncope. If injected into the ventricular cavity in cardiac resuscitation, it must not be injected into the myocardial tissue.
Pregnancy: Animal reproduction studies have not been conducted with calcium chloride. It also is not known whether calcium chloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Calcium chloride should be given to a pregnant woman only if clearly needed.
-
ADVERSE REACTIONS
Rapid injection may cause the patient to complain of tingling sensations, a calcium taste, a sense of oppression or “heat wave”.
Injections of calcium chloride are accompanied by peripheral vasodilatation as well as a local “burning” sensation and there may be a moderate fall in blood pressure.
Should perivascular infiltration occur, I.V. administration at that site should be discontinued at once. Local infiltration of the affected area with 1% procaine hydrochloride, to which hyaluronidase may be added, will often reduce venospasm and dilute the calcium remaining in the tissues locally. Local application of heat may also be helpful.
- DRUG ABUSE & DEPENDENCE
-
DOSAGE & ADMINISTRATION
10% Calcium Chloride Injection, USP is administered only by slow intravenous injection (not to exceed 1 mL/min) and/or in cardiac resuscitation, by injection into the ventricular cavity. It must not be injected into the myocardium.
The usual precautions for intravenous therapy should be observed. If time permits, the solution should be warmed to body temperature. The injection should be halted if the patient complains of any discomfort; it may be resumed when symptoms disappear. Following injection, the patient should remain recumbent for a short time.
The usual adult dosage in hypocalcemic disorders ranges from 500 mg to 1 g (5 to 10 mL) at intervals of 1 to 3 days, depending on the response of the patient and/or results of serum calcium determinations. Repeated injections may be required because of rapid excretion of calcium.
In magnesium intoxication, an initial adult dose of 500 mg (5 mL) should be administered promptly and the patient observed for signs of recovery before further doses are given.
In hyperkalemic ECG disturbances of cardiac function, the dosage of calcium chloride injection should be titrated by constant monitoring of ECG changes during administration.
In cardiac resuscitation, the usual adult dosage ranges from 500 mg to 1 g (5 to 10 mL) intravenously, or from 200 to 800 mg (2 to 8 mL) when injected into the ventricular cavity.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. See PRECAUTIONS.
To prevent needle-stick injuries, needles should not be recapped, purposely bent or broken by hand.
-
OVERDOSAGE
oo rapid injection may produce lowering of blood pressure and cardiac syncope. Persistent hypercalcemia from overdosage of calcium is unlikely because of rapid excretion. In the event of untoward effects from excessive calcium administration, the drug should be discontinued promptly, the patient re-evaluated and appropriate countermeasures instituted, if necessary. See PRECAUTIONS and ADVERSE REACTIONS.
-
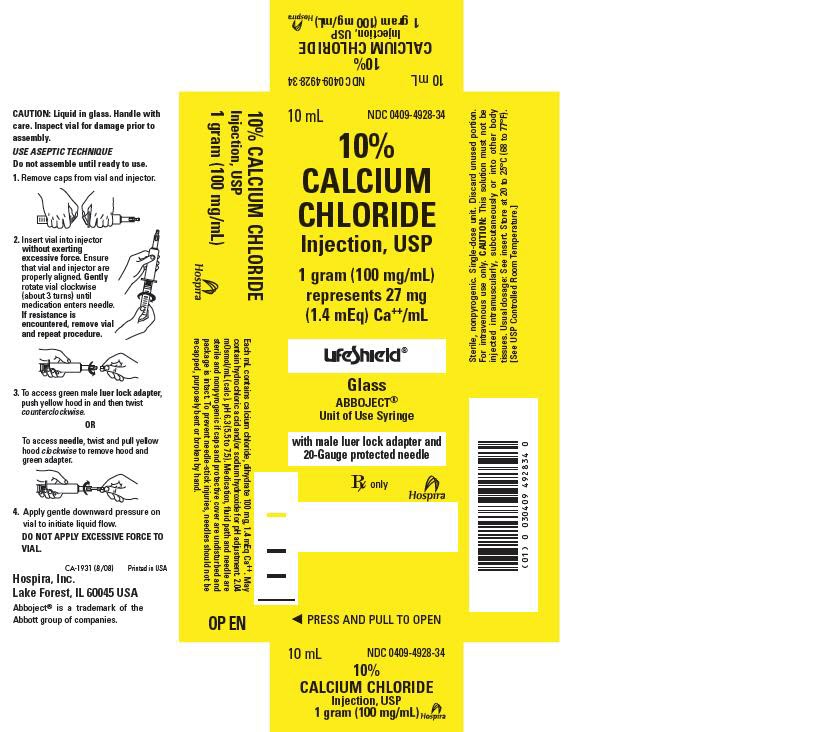
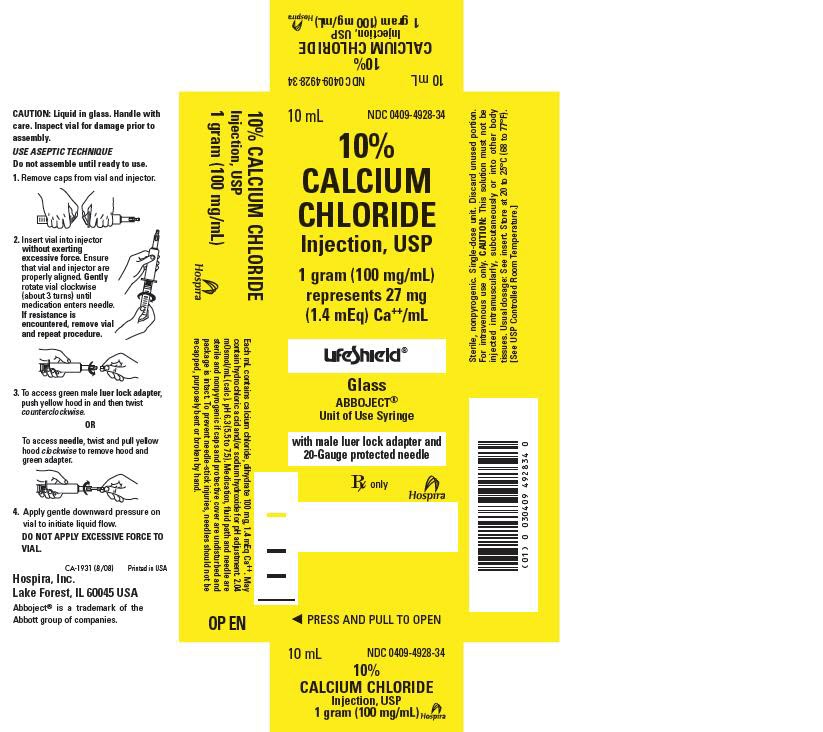
HOW SUPPLIED
10% CALCIUM CHLORIDE INJECTION is supplied in the following dosage forms.
NDC 51662-1209-1
10% CALCIUM CHLORIDE INJECTION, USP 1gram (100mg/mL) SYRNDC 51662-1209-2
10% CALCIUM CHLORIDE INJECTION, USP 1gram (100mg/mL) SYR, 1 SYRINGE PER POUCHNDC 51662-1209-3
10% CALCIUM CHLORIDE INJECTION, USP 1gram (100mg/mL) SYR, 1 SYRINGE PER POUCH, 10 POUCHES PER CASE
HF Acquisition Co LLC, DBA HealthFirst
Mukilteo, WA 98275Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Abboject® is a trademark of the Abbott group of companies
LIFESHIELD® is the trademark of ICU Medical, Inc.
-
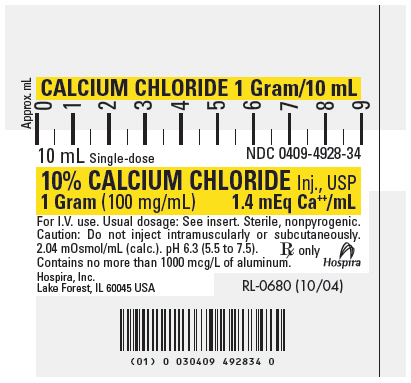
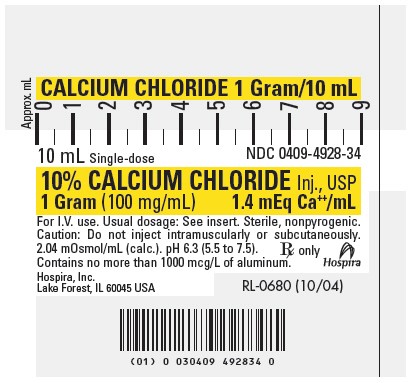
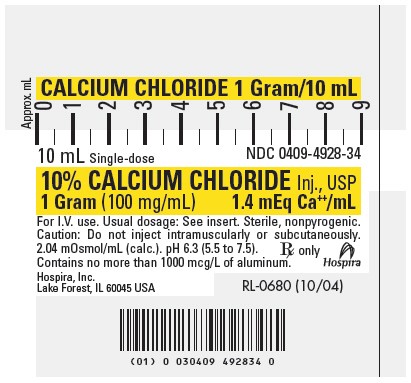
PRINCIPLE DISPLAY PANEL, SYRINGE
CALCIUM CHLORIDE 1 Gram/10 mL
10 mL Single-dose
NDC 0409-4928-3410% CALCIUM CHLORIDE Inj., USP
1 Gram (100 mg/mL)
1.4 mEq Ca++/mLFor I.V. use. Usual dosage: See insert. Sterile, nonpyrogenic.
Caution: Do not inject intramuscularly or subcutaneously.
2.04 mOsmol/mL (calc.). pH 6.3 (5.5 to 7.5).
Contains no more than 1000 mcg/L of aluminum.Rx only
Hospira
Hospira, Inc.
Lake Forest, IL 60045 USARL-0680 (10/04)
- PRINCIPLE DISPLAY PANEL, CARTON
- PRINCIPLE DISPLAY PANEL, SERIALIZED LABEL
- PRINCIPAL DISPLAY PANEL - NDC 51662-1209-2 POUCH LABELING
- PRINCIPAL DISPLAY PANEL - NDC 51662-1209-3 CASE LABELING
-
INGREDIENTS AND APPEARANCE
CALCIUM CHLORIDE
calcium chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51662-1209(NDC:0409-4928) Route of Administration INTRAVENOUS, INTRAVENTRICULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51662-1209-1 1 in 1 CARTON 09/13/2018 1 10 mL in 1 SYRINGE, GLASS; Type 1: Convenience Kit of Co-Package 2 NDC:51662-1209-3 10 in 1 CASE 12/12/2022 2 NDC:51662-1209-2 1 in 1 POUCH 2 1 in 1 CARTON 2 10 mL in 1 SYRINGE, GLASS; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/13/2018 Labeler - HF Acquisition Co LLC, DBA HealthFirst (045657305) Registrant - HF Acquisition Co LLC, DBA HealthFirst (045657305) Establishment Name Address ID/FEI Business Operations HF Acquisition Co LLC, DBA HealthFirst 045657305 relabel(51662-1209)