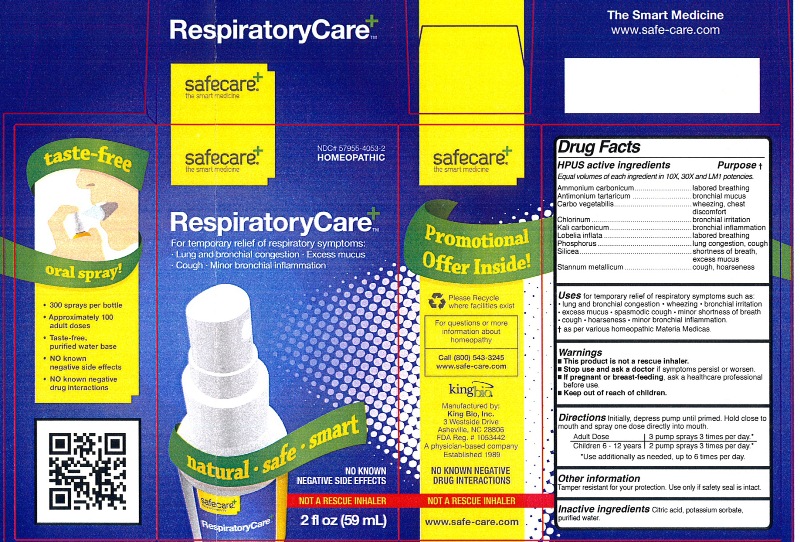
Label: RESPIRATORYCARE- ammonium carbonicum, antimonium tartaricum, carbo vegetabilis, chlorinum, kali carbonicum, lobelia inflata, phosphorus, silicea, and stannum metallicum liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 57955-4053-2 - Packager: King Bio Inc
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated June 5, 2012
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Inactive Ingredients
- Directions
-
Purpose
HPUS active ingredients Purpose
Equal volumes of each ingredient in 10X, 30X and LM1
Ammonium carbonicum....................................................labored breathing
Antimonium tartaricum.....................................................bronchial mucus
Carbo vegetabilis.............................................................wheezing, chest discomfort
Chlorinum.......................................................................bronchial irritation
Kali carbonicum..............................................................bronchial inflammation
Lobelia inflata..................................................................labored breathing
Phosphorus....................................................................lung congestion, cough
Silicea............................................................................shortness of breath, excess mucus
Stannum metallicum........................................................cough, hoarseness
Reference image respiratorycare.jpg
-
Warnings
- This product is not a rescue inhaler.
- Stop use and ask a doctor if symptoms persist or worsen.
- If pregnant or breast-feeding, ask a healthcare professional before use.
- Keep out of reach of children.
Other information: Tamper resistant for your protection. Use only if safety seal is intact.
Reference image respiratorycare.jpg
- KEEP OUT OF REACH OF CHILDREN
- Indications and Usage
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RESPIRATORYCARE
ammonium carbonicum, antimonium tartaricum, carbo vegetabilis, chlorinum, kali carbonicum, lobelia inflata, phosphorus, silicea, and stannum metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57955-4053 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMMONIUM CARBONATE (UNII: NJ5VT0FKLJ) (AMMONIUM CATION - UNII:54S68520I4) AMMONIUM CARBONATE 10 [hp_X] in 59 mL ANTIMONY POTASSIUM TARTRATE (UNII: DL6OZ476V3) (ANTIMONY - UNII:9IT35J3UV3) ANTIMONY POTASSIUM TARTRATE 10 [hp_X] in 59 mL ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 10 [hp_X] in 59 mL CHLORINE (UNII: 4R7X1O2820) (CHLORINE - UNII:4R7X1O2820) CHLORINE 10 [hp_X] in 59 mL POTASSIUM CARBONATE (UNII: BQN1B9B9HA) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CARBONATE 10 [hp_X] in 59 mL LOBELIA INFLATA (UNII: 9PP1T3TC5U) (LOBELIA INFLATA - UNII:9PP1T3TC5U) LOBELIA INFLATA 10 [hp_X] in 59 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 10 [hp_X] in 59 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 10 [hp_X] in 59 mL TIN (UNII: 387GMG9FH5) (TIN - UNII:387GMG9FH5) TIN 10 [hp_X] in 59 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57955-4053-2 1 in 1 CARTON 1 59 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/01/2012 Labeler - King Bio Inc (617901350) Registrant - King Bio Inc (617901350) Establishment Name Address ID/FEI Business Operations King Bio Inc 617901350 manufacture