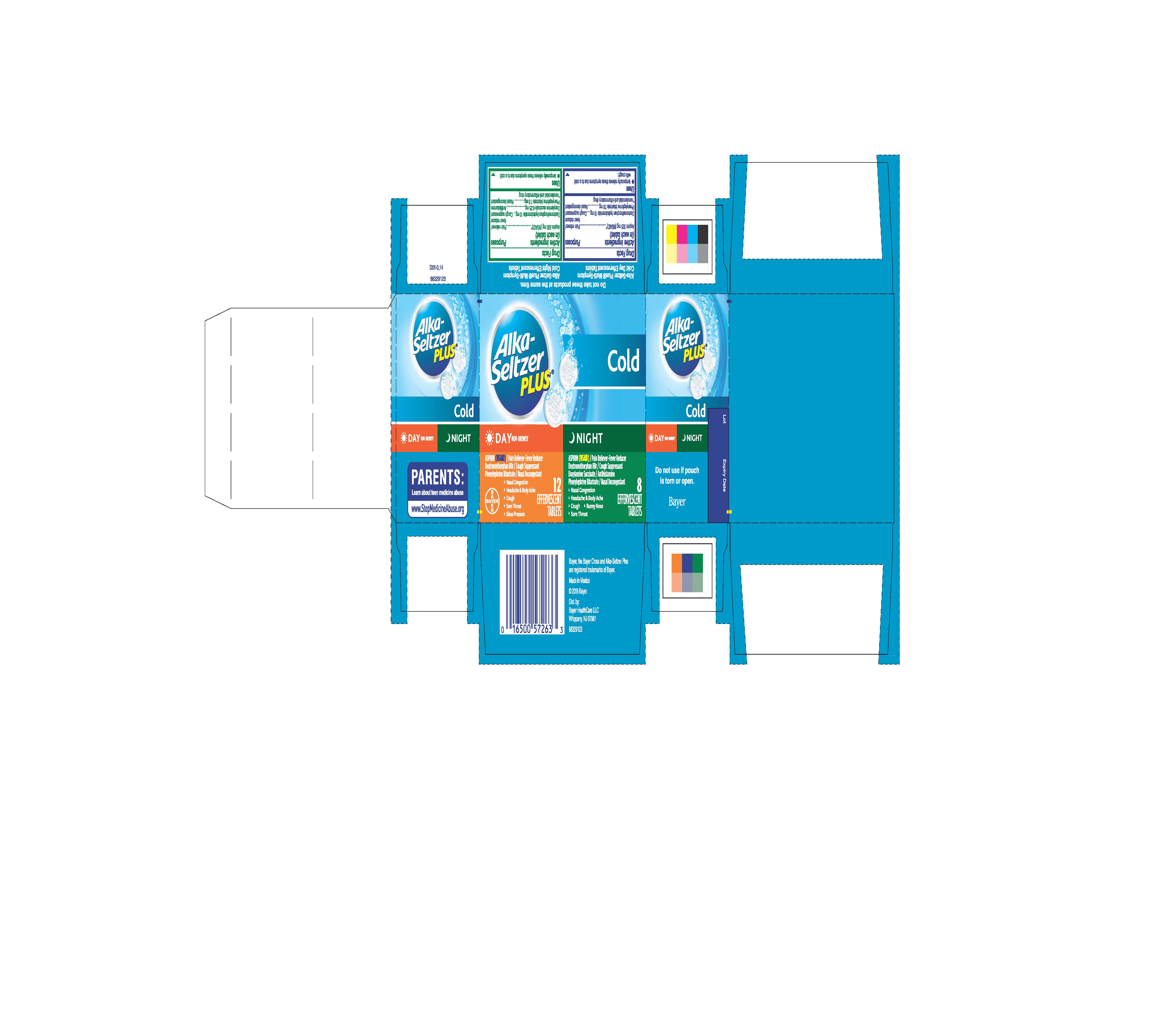
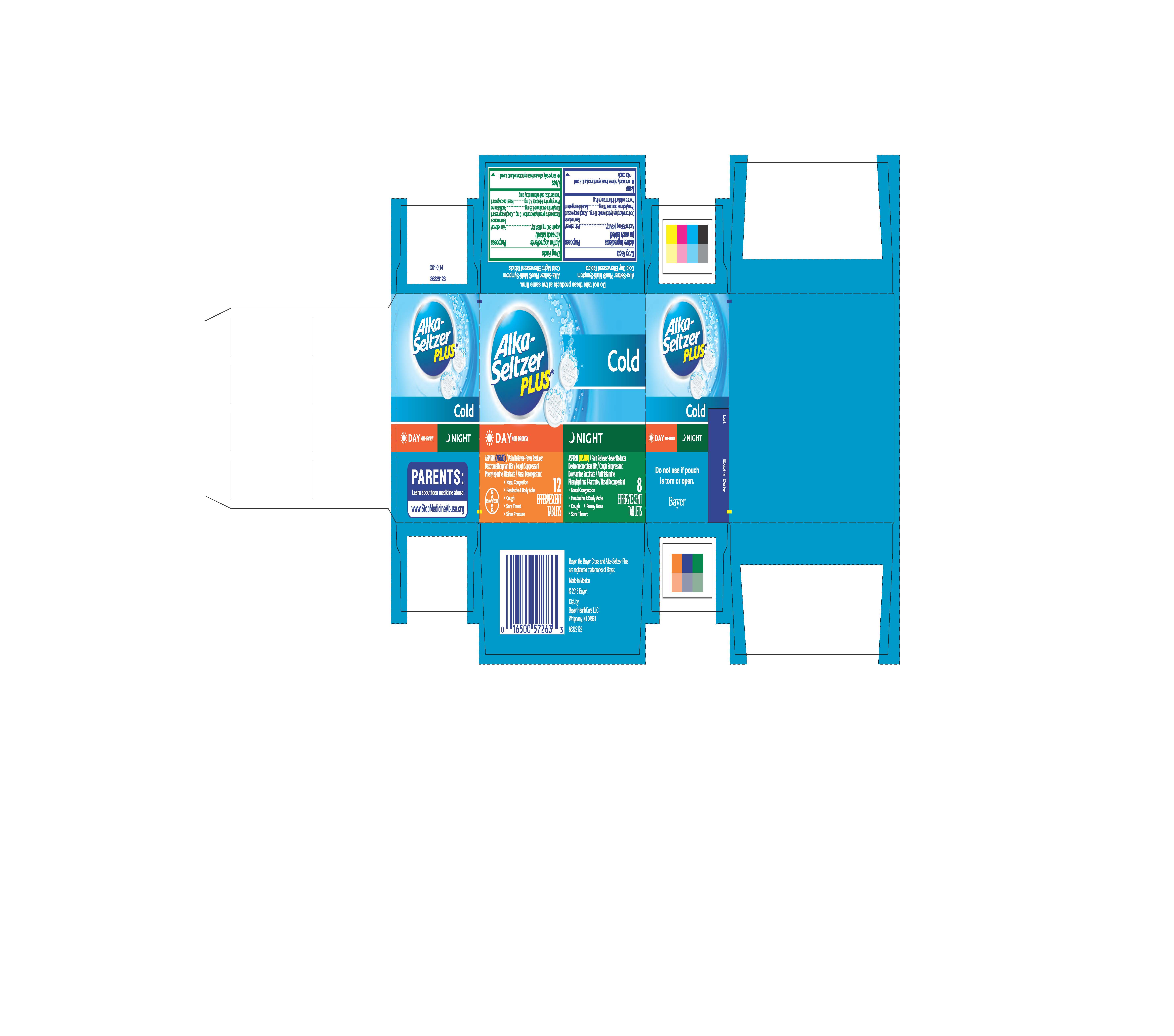
Label: ALKA-SELTZER PLUS COLD DAY AND NIGHT- aspirin, dextromethorphan hydrobromide, phenylephrine bitartrate, doxylamine succinate kit
- NDC Code(s): 0280-1550-20
- Packager: Bayer HealthCare LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Reye’s syndrome: Children and teenagers who have or are recovering from
chicken pox or flu-like symptoms should not use this product. When using this
product, if changes in behavior with nausea and vomiting occur, consult a
doctor because these symptoms could be an early sign of Reye’s syndrome, a
rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
● hives ● facial swelling ● asthma (wheezing) ● shock
Stomach bleeding warning: This product contains an NSAID, which may
cause severe stomach bleeding. The chance is higher if you
● are age 60 or older
● have had stomach ulcers or bleeding problems
● take a blood thinning (anticoagulant) or steroid drug
● take other drugs containing prescription or nonprescription NSAIDs
(aspirin, ibuprofen, naproxen, or others)
● have 3 or more alcoholic drinks every day while using this product
● take more or for a longer time than directed
Sore throat warning: If sore throat is severe, persists for more than 2 days,
is accompanied or followed by fever, headache, rash, nausea, or vomiting,
consult a doctor promptly.
-
DO NOT USE
Do not use
● if you are allergic to aspirin or any other pain reliever/fever reducer
● if you are now taking a prescription monoamine oxidase inhibitor (MAOI)
(certain drugs for depression, psychiatric, or emotional conditions, or
Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you
do not know if your prescription drug contains an MAOI, ask a doctor or
pharmacist before taking this product.
● if you have ever had an allergic reaction to this product or any of its
ingredients
● in children under 12 years of age
-
ASK DOCTOR
Ask a doctor before use if
● stomach bleeding warning applies to you
● you have a history of stomach problems, such as heartburn
● you have high blood pressure, heart disease, liver cirrhosis, or kidney
disease
● you are taking a diuretic
● you have
● asthma ● thyroid disease ● diabetes
● cough with excessive phlegm (mucus)
● difficulty in urination due to enlargement of the prostate gland
● persistent or chronic cough such as occurs with smoking, asthma,
or emphysema
● a sodium-restricted diet
- ASK DOCTOR/PHARMACIST
- WHEN USING
-
STOP USE
Stop use and ask a doctor if
● an allergic reaction occurs. Seek medical help right away.
● you experience any of the following signs of stomach bleeding
● feel faint ● vomit blood ● have bloody or black stools
● have stomach pain that does not get better
● pain, cough, or nasal congestion gets worse or lasts more than 7 days
● fever gets worse or lasts more than 3 days
● redness or swelling is present
● new symptoms occur
● ringing in the ears or loss of hearing occurs
● cough comes back or occurs with rash or headache that lasts. These could be signs of a serious condition.
● nervousness, dizziness, or sleeplessness occurs
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- INACTIVE INGREDIENT
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- Uses
-
WARNINGS
Warnings
Reye’s syndrome: Children and teenagers who have or are recovering
from chicken pox or flu-like symptoms should not use this product. When
using this product, if changes in behavior with nausea and vomiting occur,
consult a doctor because these symptoms could be an early sign of Reye’s
syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may
include:
● hives ● facial swelling ● asthma (wheezing) ● shock
Stomach bleeding warning: This product contains an NSAID, which may
cause severe stomach bleeding. The chance is higher if you
● are age 60 or older
● have had stomach ulcers or bleeding problems
● take a blood thinning (anticoagulant) or steroid drug
● take other drugs containing prescription or nonprescription NSAIDs
(aspirin, ibuprofen, naproxen, or others)
● have 3 or more alcoholic drinks every day while using this product
● take more or for a longer time than directed
Sore throat warning: If sore throat is severe, persists for more than
2 days, is accompanied or followed by fever, headache, rash, nausea,
or vomiting, consult a doctor promptly.
Do not use to sedate children.
-
DO NOT USE
Do not use
● if you are allergic to aspirin or any other pain reliever/fever reducer
● if you are now taking a prescription monoamine oxidase inhibitor
(MAOI) (certain drugs for depression, psychiatric, or emotional
conditions, or Parkinson's disease), or for 2 weeks after stopping
the MAOI drug. If you do not know if your prescription drug contains
an MAOI, ask a doctor or pharmacist before taking this product.
● if you have ever had an allergic reaction to this product or any of its
ingredients
● in children under 12 years of age
-
ASK DOCTOR
Ask a doctor before use if
● stomach bleeding warning applies to you
● you have a history of stomach problems, such as heartburn
● you have high blood pressure, heart disease, liver cirrhosis, or
kidney disease
● you are taking a diuretic
● you have
● asthma ● diabetes ● thyroid disease ● glaucoma
● cough with excessive phlegm (mucus)
● a breathing problem such as emphysema or chronic bronchitis
● difficulty in urination due to enlargement of the prostate gland
● persistent or chronic cough such as occurs with smoking, asthma,
or emphysema
● a sodium-restricted diet
- ASK DOCTOR/PHARMACIST
- WHEN USING
-
STOP USE
Stop use and ask a doctor if
● an allergic reaction occurs. Seek medical help right away.
● you experience any of the following signs of stomach bleeding
● feel faint ● vomit blood ● have bloody or black stools
● have stomach pain that does not get better
● pain, cough, or nasal congestion gets worse or lasts more than 7 days
● fever gets worse or lasts more than 3 days
● redness or swelling is present
● new symptoms occur
● ringing in the ears or loss of hearing occurs
● cough comes back or occurs with rash or headache that lasts. These
could be signs of a serious condition.
● nervousness, dizziness, or sleeplessness occurs
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- INACTIVE INGREDIENT
- Questions or comments?
-
Package Display Label
Alka-Seltzer
PLUS®
DAY NON-DROWSY
ASPIRIN (NSAID) / Pain Reliever-Fever Reducer
Dextromethorphan HBr / Cough Suppressant
Phenylephrine Bitartrate / Nasal Decongestant
- Nasal Congestion
- Headache & Body Ache
- Cough
- Sore Throat
- Sinus Pressure
12 EFFERVESCENT TABLETS
NIGHT
ASPIRIN (NSAID) / Pain Reliever-Fever Reducer
Dextromethorphan HBr / Cough Suppressant
Doxylamine Succinate / Antihistamine
Phenylephrine Bitartrate / Nasal Decongestant
- Nasal Congestion
- Headache & Body Ache
- Cough
- Runny nose
- Sore Throat
8 EFFERVESCENT TABLETS

-
INGREDIENTS AND APPEARANCE
ALKA-SELTZER PLUS COLD DAY AND NIGHT
aspirin, dextromethorphan hydrobromide, phenylephrine bitartrate, doxylamine succinate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0280-1550 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0280-1550-20 1 in 1 CARTON; Type 0: Not a Combination Product 09/14/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 POUCH 12 Part 2 4 POUCH 8 Part 1 of 2 ALKA-SELTZER PLUS COLD DAY
aspirin, dextromethorphan hydrobromide, phenylephrine bitartrate tablet, effervescentProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE BITARTRATE (UNII: 27O3Q5ML57) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE BITARTRATE 7.8 mg ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 325 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg Inactive Ingredients Ingredient Name Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) CALCIUM SILICATE (UNII: S4255P4G5M) SODIUM BENZOATE (UNII: OJ245FE5EU) ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) DOCUSATE SODIUM (UNII: F05Q2T2JA0) MANNITOL (UNII: 3OWL53L36A) POVIDONE (UNII: FZ989GH94E) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ASPARTAME (UNII: Z0H242BBR1) DIMETHICONE (UNII: 92RU3N3Y1O) Product Characteristics Color white Score no score Shape ROUND Size 25mm Flavor CITRUS Imprint Code ASP;DAY Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6 in 1 CARTON 1 2 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 09/14/2018 Part 2 of 2 ALKA-SELTZER PLUS COLD NIGHT
aspirin, dextromethorphan hydrobromide, phenylephrine bitartrate, doxylamine succinate tablet, effervescentProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 6.25 mg ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 500 mg PHENYLEPHRINE BITARTRATE (UNII: 27O3Q5ML57) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE BITARTRATE 7.8 mg Inactive Ingredients Ingredient Name Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) CALCIUM SILICATE (UNII: S4255P4G5M) ASPARTAME (UNII: Z0H242BBR1) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) DOCUSATE SODIUM (UNII: F05Q2T2JA0) POVIDONE (UNII: FZ989GH94E) SODIUM BENZOATE (UNII: OJ245FE5EU) MANNITOL (UNII: 3OWL53L36A) DIMETHICONE (UNII: 92RU3N3Y1O) Product Characteristics Color white Score no score Shape ROUND Size 25mm Flavor LEMON Imprint Code ASP;NT Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 4 in 1 CARTON 1 2 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 09/14/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 09/14/2018 Labeler - Bayer HealthCare LLC. (112117283)