Label: NIOXIN SCALP RECOVERY SOOTHING SERUM- pyrithione zinc lotion/shampoo
- NDC Code(s): 69282-006-10, 69282-006-50
- Packager: The Wella Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 21, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
water, stearamidopropyl dimethylamine, cetyl alcohol, hydroxyethylcellulose, quaternium-18, benzyl alcohol, phenoxyethanol, stearyl alcohol, PEG-2M, dimethicone, cetearyl alcohol, methylparaben, propylparaben, fragrance, glyceryl stearate, oleyl alcohol, citric acid, polysorbate 60, mentha piperita (peppermint) oil, menthol, mentha arvensis leaf oil, yeast extract, camellia sinensis leaf extract, lecithin, PPG-26-buteth-26, saccharomyces/magnesium ferment, dimethyl isosorbide, carnitine HCL, PEG/PPG-18/18 dimethicone, ethoxydiglycol, oryza sativa (rice) bran, PEG-40 hydrogenated castor oil, biotin/folic acid/cyanocobalamin/niacinamide/pantothenic acid/pyridoxine/riboflavin/thiamine/yeast polypeptides, saccharomyces/iron ferment, saccharomyces/copper ferment, saccharomyces/silicon ferment, saccharomyces/zinc ferment, acacia senegal gum, ubiquinone.
- Questions?
- SPL UNCLASSIFIED SECTION
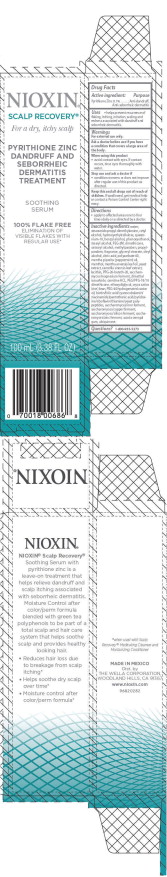
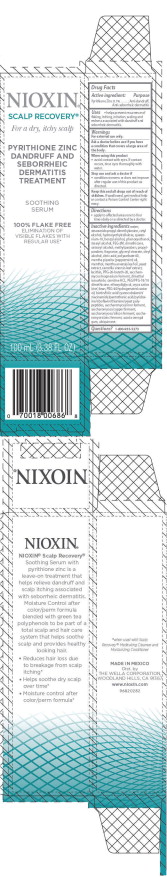
- PRINCIPAL DISPLAY PANEL - 100 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
NIOXIN SCALP RECOVERY SOOTHING SERUM
pyrithione zinc lotion/shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69282-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC 0.1 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) STEARAMIDOPROPYL DIMETHYLAMINE (UNII: K7VEI00UFR) CETYL ALCOHOL (UNII: 936JST6JCN) QUATERNIUM-18 (UNII: O7757NO1VL) BENZYL ALCOHOL (UNII: LKG8494WBH) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) POLYETHYLENE OXIDE 100000 (UNII: V46Y6OJ5QB) DIMETHICONE (UNII: 92RU3N3Y1O) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) OLEYL ALCOHOL (UNII: 172F2WN8DV) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POLYSORBATE 60 (UNII: CAL22UVI4M) PEPPERMINT OIL (UNII: AV092KU4JH) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) MENTHA ARVENSIS LEAF OIL (UNII: 1AEY1M553N) YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PPG-26-BUTETH-26 (UNII: 2II1K6TZ4P) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) LEVOCARNITINE HYDROCHLORIDE (UNII: J3Y5E6IKS3) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) RICE BRAN (UNII: R60QEP13IC) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) ACACIA (UNII: 5C5403N26O) UBIDECARENONE (UNII: EJ27X76M46) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69282-006-50 1 in 1 CARTON 07/01/2016 1 50 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:69282-006-10 1 in 1 CARTON 07/01/2016 2 100 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 07/01/2016 Labeler - The Wella Corporation (001399815) Registrant - Coty US LLC (039056361) Establishment Name Address ID/FEI Business Operations Thibiant International, Inc. 118542196 manufacture(69282-006)