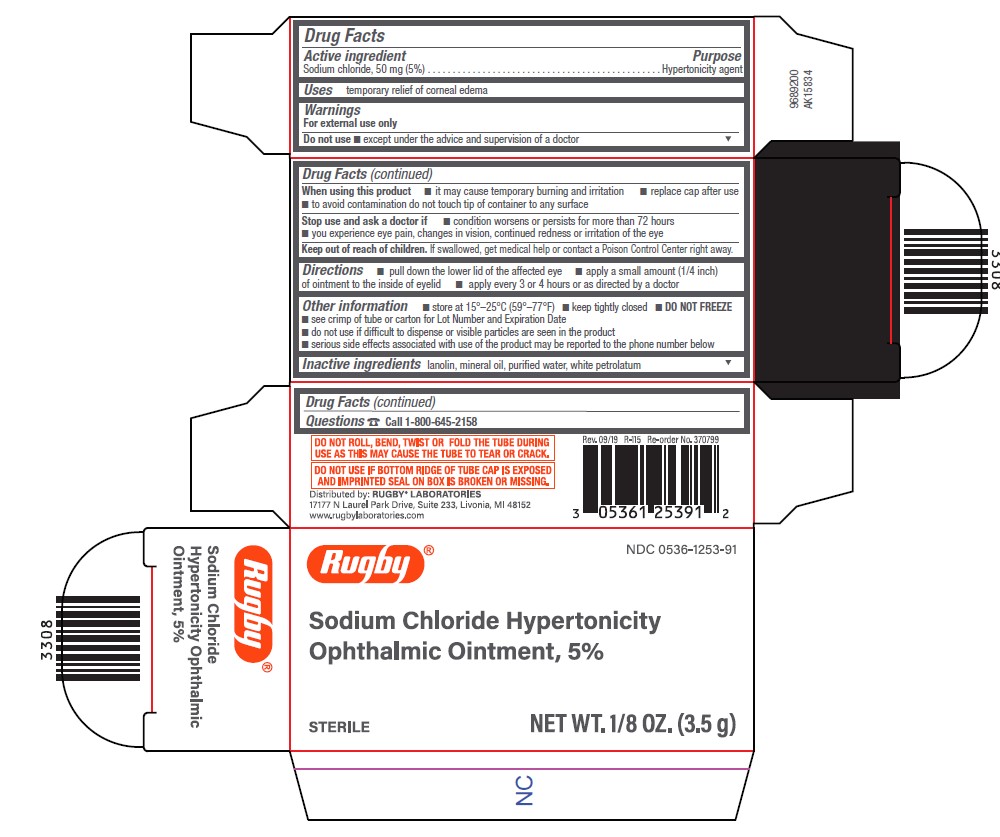
Label: SODIUM CHLORIDE HYPERTONICITY OPHTHALMIC- sodium chloride ointment
- NDC Code(s): 0536-1253-91
- Packager: Rugby Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 22, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use except under the advice and supervision of a doctor
When using this product
- •
- it may cause temporary burning and irritation
- •
- replace cap after use
- •
- to avoid contamination do not touch tip of container to any surface
Stop use and ask a doctor if
- •
- condition worsens or persists for more than 72 hours
- •
- you experience eye pain, changes in vision, continued redness or irritation of the eye
- Keep Out of Reach of Children
- Directions
-
Other information
- •
- store at 15° - 25°C (59° - 77°F)
- •
- keep tightly closed
- •
- DO NOT FREEZE
- •
- see crimp of tube or carton for Lot Number and Expiration Date
- •
- do not use if difficult to dispense or visible particles are seen in the product
- •
- serious side effects associated with use of the product may be reported to the phone number below
- Inactive ingredients
- Questions [phone icon]
- Package/Label Principal Display Panel Carton
-
INGREDIENTS AND APPEARANCE
SODIUM CHLORIDE HYPERTONICITY OPHTHALMIC
sodium chloride ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0536-1253 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength LANOLIN (UNII: 7EV65EAW6H) MINERAL OIL (UNII: T5L8T28FGP) WATER (UNII: 059QF0KO0R) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0536-1253-91 1 in 1 CARTON 11/06/2020 1 3.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 11/06/2020 Labeler - Rugby Laboratories (079246066) Establishment Name Address ID/FEI Business Operations Bausch & Lomb Incorporated 079587625 MANUFACTURE(0536-1253)