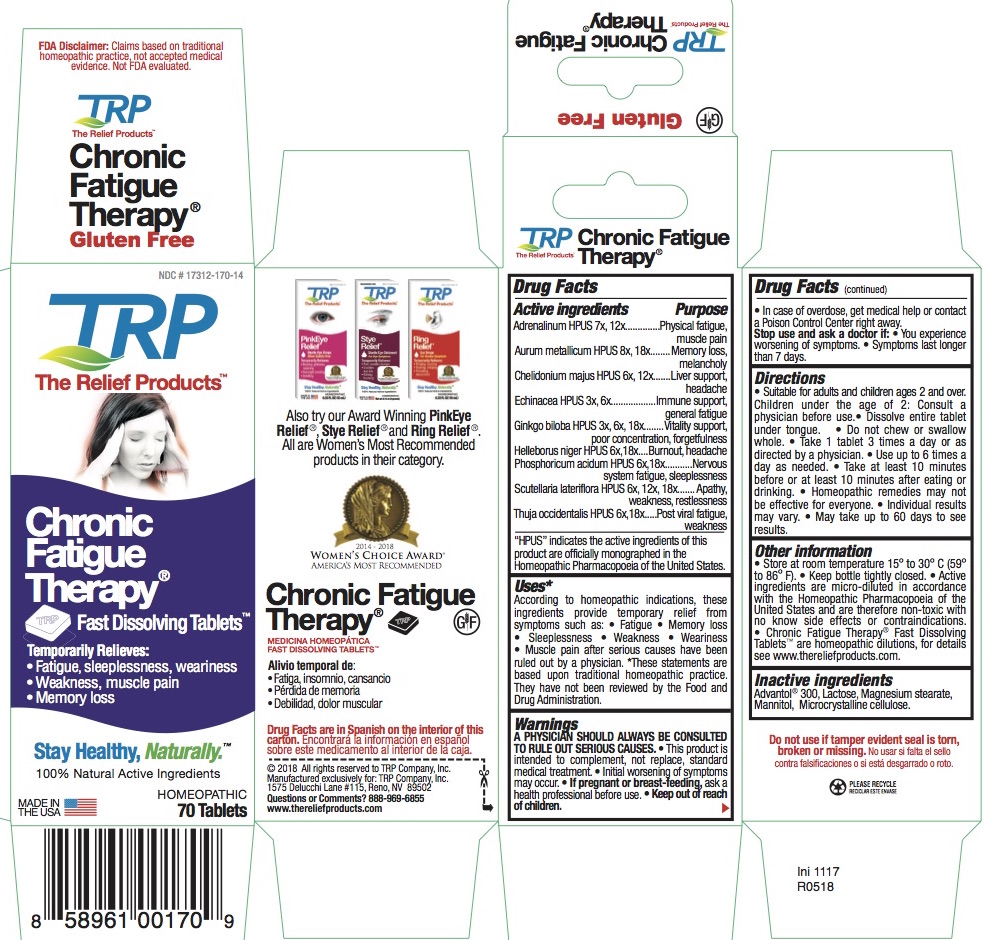
Label: CHRONIC FATIGUE THERAPY- epinephrine, gold, chelidonium majus, echinacea, ginkgo, helleborus niger, phosphoric acid, scutellaria lateriflora, thuja occidentalis tablet, orally disintegrating
- NDC Code(s): 17312-170-14
- Packager: TRP Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated August 20, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Adrenalinum HPUS 7x, 12x
Aurum metallicum HPUS 8x, 18x
Chelidonium majus HPUS 6x, 12x
Echinacea HPUS 3x, 6x
Ginkgo biloba HPUS 3x, 6x, 18x
Helleborus niger HPUS 6x,18x
Phosphoricum acidum HPUS 6x,18x
Scutellaria lateriflora HPUS 6x, 12x, 18x
Thuja occidentalis HPUS 6x,18x
HPUS indicates the active ingredients are in the Homeopathic Pharmacopoeia of the United States.
-
PURPOSE
Adrenalinum HPUS..................Physical fatigue, muscle pain
Aurum metallicum HPUS .........Memory loss, melancholy
Chelidonium majus HPUS........Liver support, headache
Echinacea HPUS......................Immune support, general fatigue
Ginkgo biloba HPUS.................Vitality support, poor concentration, forgetfulness
Helleborus niger HPUS.............Burnout, headache
Phosphoricum acidum HPUS....Nervous system fatigue, sleeplessness
Scutellaria lateriflora HPUS...... Apathy, weakness, restlessness
Thuja occidentalis HPUS...........Post viral fatigue, weakness -
Uses
According to homeopathic indications, these ingredients provide temporary relief from symptoms such as: • Fatigue • Memory loss • Sleeplessness • Weakness • Weariness • Muscle pain after serious causes have been ruled out by a physician.
*These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
- Warnings
- PREGNANCY OR BREAST FEEDING
- Keep out of reach of children
-
Directions
- Suitable for adults and children ages 2 and over. Children under the age of 2: Consult a physician before use.
- Dissolve entire tablet under tongue.
- Do not chew or swallow whole. • Take 1 tablet 3 times a day or as directed by a physician.
- Use up to 6 times a day as needed.
- Take at least 10 minutes before or at least 10 minutes after eating or drinking.
- Homeopathic remedies may not be effective for everyone. •
- Individual results may vary.
- May take up to 60 days to see results.
-
Other information
- Store at room temperature 15o to 30o C (59o to 86o F).
- Keep bottle tightly closed. • Active ingredients are micro-diluted in accordance with the Homeopathic Pharmacopoeia of the United States and are therefore non-toxic with no know side effects or contraindications.
- Chronic Fatigue Therapy® Fast Dissolving TabletsTM are homeopathic dilutions, for details see www.thereliefproducts.com.
- Inactive Ingredients
- Do not use if tamper evident seal is torn, broken or missing.
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CHRONIC FATIGUE THERAPY
epinephrine, gold, chelidonium majus, echinacea, ginkgo, helleborus niger, phosphoric acid, scutellaria lateriflora, thuja occidentalis tablet, orally disintegratingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17312-170 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GINKGO (UNII: 19FUJ2C58T) (GINKGO - UNII:19FUJ2C58T) GINKGO 3 [hp_X] CHELIDONIUM MAJUS (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS - UNII:7E889U5RNN) CHELIDONIUM MAJUS 6 [hp_X] EPINEPHRINE (UNII: YKH834O4BH) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 7 [hp_X] PHOSPHORIC ACID (UNII: E4GA8884NN) (PHOSPHORIC ACID - UNII:E4GA8884NN) PHOSPHORIC ACID 6 [hp_X] ECHINACEA, UNSPECIFIED (UNII: 4N9P6CC1DX) (ECHINACEA, UNSPECIFIED - UNII:4N9P6CC1DX) ECHINACEA, UNSPECIFIED 3 [hp_X] GOLD (UNII: 79Y1949PYO) (GOLD - UNII:79Y1949PYO) GOLD 8 [hp_X] HELLEBORUS NIGER ROOT (UNII: 608DGJ6815) (HELLEBORUS NIGER ROOT - UNII:608DGJ6815) HELLEBORUS NIGER ROOT 6 [hp_X] SCUTELLARIA LATERIFLORA (UNII: 7BP4DH5PDC) (SCUTELLARIA LATERIFLORA - UNII:7BP4DH5PDC) SCUTELLARIA LATERIFLORA 6 [hp_X] THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 6 [hp_X] Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SORBITOL (UNII: 506T60A25R) CROSPOVIDONE (UNII: 68401960MK) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) COPOVIDONE (UNII: D9C330MD8B) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score no score Shape DIAMOND Size 13mm Flavor Imprint Code TRP Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17312-170-14 1 in 1 PACKAGE 08/20/2018 1 70 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/20/2018 Labeler - TRP Company (105185719)