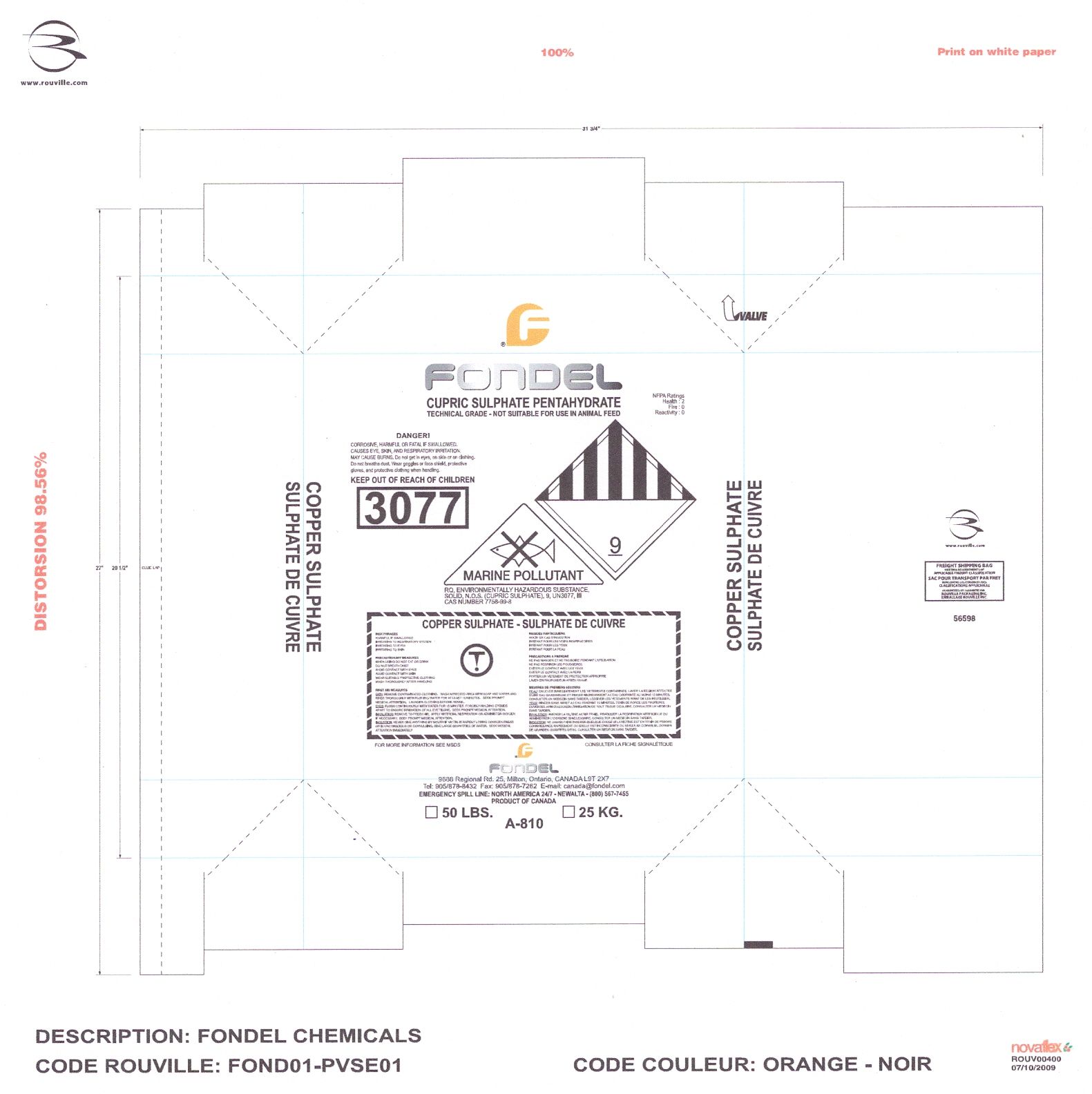
Label: CUPRIC SULFATE PENTAHYDRATE- cupric sulfate powder, for solution
- NDC Code(s): 51044-101-25
- Packager: Fondel Chemicals Ltd.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 25, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
-
DO NOT USE
Warnings:
- Copper can be toxic to sheep. Do not allow animals to eat or drink the cupric sulphate powder or hoof bath solution.
- Know the volume of the hoof bath and calculate the amount of cupric sulphate carefully.
- Do not overdose by using more cupric sulphate than what is recommended by your veterinarian.
-
WHEN USING
When Using this Product: By placing a clean water bath ahead of the treatment bath, animals will clean their hooves to some extent and keep the treatment bath clean longer. Hoof baths should only be part of an overall program that includes proper nutrition, regular hoof trimming, and hoof injury prevention.
- KEEP OUT OF REACH OF CHILDREN
- QUESTIONS
- Cupric Sulfate Labels
-
INGREDIENTS AND APPEARANCE
CUPRIC SULFATE PENTAHYDRATE
cupric sulfate powder, for solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:51044-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Cupric Sulfate (UNII: LRX7AJ16DT) (Cupric Cation - UNII:8CBV67279L) Cupric Cation 0.4 kg in 1.6 kg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51044-101-25 25 kg in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/2008 Labeler - Fondel Chemicals Ltd. (201786790) Registrant - Fondel Chemicals Ltd. (201786790) Establishment Name Address ID/FEI Business Operations Fondel Chemicals Ltd. 201786790 manufacture, label