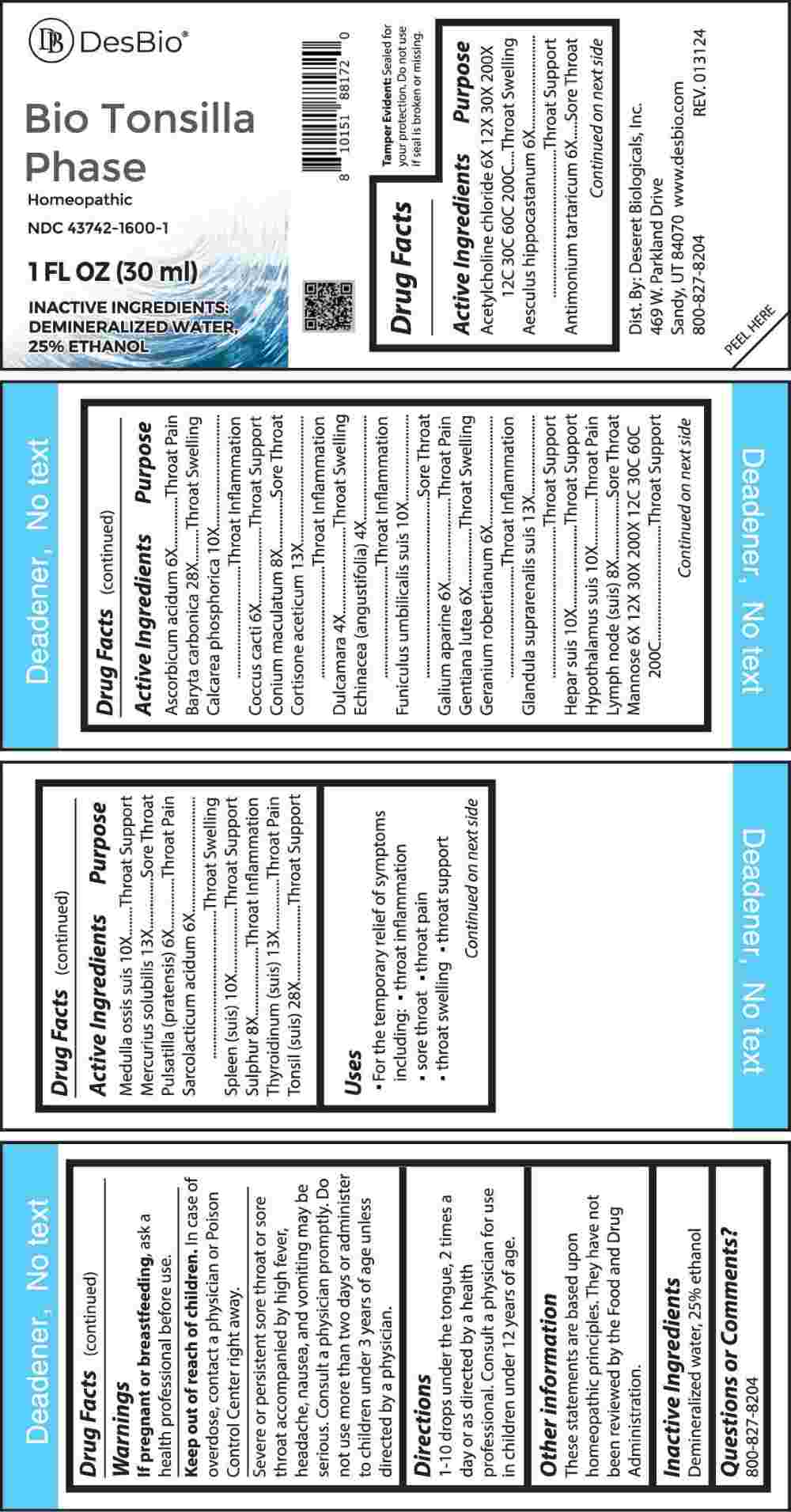
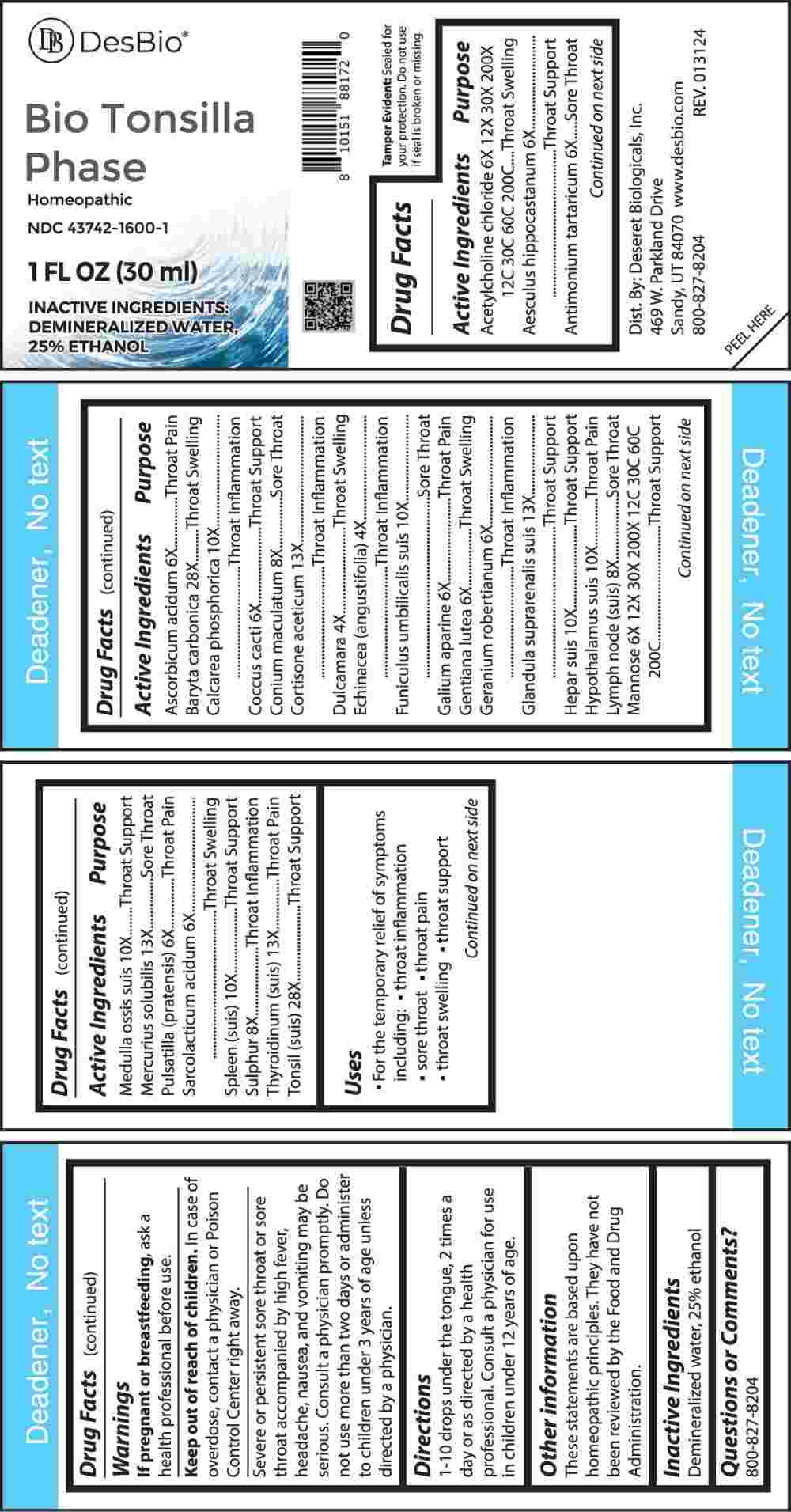
Label: BIO TONSILLA PHASE (dulcamara, echinacea (angustifolia), aesculus hippocastanum, antimonium tartaricum, ascorbicum acidum, coccus cacti, galium aparine, gentiana lutea, geranium robertianum, pulsatilla (pratensis), sarcolacticum acidum, acetylcholine chloride, mannose, conium maculatum, lymph node (suis), sulphur, calcarea phosphorica, funiculus umbilicalis suis, hepar suis, hypothalamus suis, medulla ossis suis, spleen (suis), cortisone aceticum, glandula suprarenalis suis, mercurius solubilis, thyroidinum- suis, liquid
- NDC Code(s): 43742-1600-1
- Packager: Deseret Biologicals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated May 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTS:
Acetylcholine Chloride 6X, 12X, 30X, 200X, 12C, 30C, 60C, 200C, Aesculus Hippocastanum 6X, Antimonium Tartaricum 6X, Ascorbicum Acidum 6X, Baryta Carbonica 28X, Calcarea Phosphorica 10X, Coccus Cacti 6X, Conium Maculatum 8X, Cortisone Aceticum 8X, Dulcamara 4X, Echinacea (Angustifolia) 4X, Funiculus Umbilicalis Suis 10X, Galium Aparine 6X, Gentiana Lutea 6X, Geranium Robertianum 6X, Glandula Suprarenalis Suis 13X, Hepar Suis 10X, Hypothalamus Suis 10X, Lymph Node (Suis) 8X, Mannose 6X, 12X, 30X, 200X, 12C, 30C, 60C, 200C, Medulla Ossis Suis 10X, Mercurius Solubilis 13X, Pulsatilla (Pratensis) 6X, Sarcolacticum Acidum 6X, Spleen (Suis) 10X, Sulphur 8X, Thyroidinum (Suis) 13X, Tonsil Suis 28X.
-
PURPOSE:
Acetylcholine Chloride – Throat Swelling, Aesculus Hippocastanum – Throat Support, Antimonium Tartaricum – Sore Throat, Ascorbicum Acidum – Throat Pain, Baryta Carbonica – Throat Swelling, Calcarea Phosphorica – Throat Inflammation, Coccus Cacti – Throat Support, Conium Maculatum – Sore Throat, Cortisone Aceticum – Throat Inflammation, Dulcamara – Throat Swelling, Echinacea (Angustifolia) – Throat Inflammation, Funiculus Umbilicalis – Sore Throat, Galium Aparine – Throat Pain, Gentiana Lutea – Throat Swelling, Geranium Robertianum – Throat Swelling, Glandula Suprarenalis Suis – Throat Support, Hepar Suis – Throat Support, Hypothalamus Suis – Throat Support, Lymph Node (Suis) – Sore Throat, Mannose – Throat Support, Medulla Ossis Suis – Throat Support, Mercurius Solubilis – Sore Throat, Pulsatilla (Pratensis) – Throat Pain, Sarcolacticum Acidum – Throat Swelling, Spleen (Suis) – Throat Support, Sulphur – Throat Inflammation, Thyroidinum (Suis) – Throat Pain, Tonsil Suis – Throat Support
- USES:
-
WARNINGS:
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, contact a physician or Poison Control Center right away.
Tamper Evident: Sealed for your protection.
Do not use if seal is broken or missing.
Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult a physician promptly. Do not use more than two days or administer to children under 3 years of age unless directed by a physician.
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
BIO TONSILLA PHASE
dulcamara, echinacea (angustifolia), aesculus hippocastanum, antimonium tartaricum, ascorbicum acidum, coccus cacti, galium aparine, gentiana lutea, geranium robertianum, pulsatilla (pratensis), sarcolacticum acidum, acetylcholine chloride, mannose, conium maculatum, lymph node (suis), sulphur, calcarea phosphorica, funiculus umbilicalis suis, hepar suis, hypothalamus suis, medulla ossis suis, spleen (suis), cortisone aceticum, glandula suprarenalis suis, mercurius solubilis, thyroidinum (suis), liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43742-1600 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOLANUM DULCAMARA TOP (UNII: KPS1B1162N) (SOLANUM DULCAMARA TOP - UNII:KPS1B1162N) SOLANUM DULCAMARA TOP 4 [hp_X] in 1 mL ECHINACEA ANGUSTIFOLIA WHOLE (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA WHOLE 4 [hp_X] in 1 mL HORSE CHESTNUT (UNII: 3C18L6RJAZ) (HORSE CHESTNUT - UNII:3C18L6RJAZ) HORSE CHESTNUT 6 [hp_X] in 1 mL ANTIMONY POTASSIUM TARTRATE (UNII: DL6OZ476V3) (ANTIMONY CATION (3+) - UNII:069647RPT5) ANTIMONY POTASSIUM TARTRATE 6 [hp_X] in 1 mL ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 6 [hp_X] in 1 mL PROTORTONIA CACTI (UNII: LZB7TFX1LT) (PROTORTONIA CACTI - UNII:LZB7TFX1LT) PROTORTONIA CACTI 6 [hp_X] in 1 mL GALIUM APARINE WHOLE (UNII: Z4B6561488) (GALIUM APARINE - UNII:Z4B6561488) GALIUM APARINE WHOLE 6 [hp_X] in 1 mL GENTIANA LUTEA ROOT (UNII: S72O3284MS) (GENTIANA LUTEA ROOT - UNII:S72O3284MS) GENTIANA LUTEA ROOT 6 [hp_X] in 1 mL GERANIUM ROBERTIANUM WHOLE (UNII: R5I1HK0UBL) (GERANIUM ROBERTIANUM - UNII:R5I1HK0UBL) GERANIUM ROBERTIANUM WHOLE 6 [hp_X] in 1 mL PULSATILLA PRATENSIS WHOLE (UNII: 8E272251DI) (PULSATILLA PRATENSIS WHOLE - UNII:8E272251DI) PULSATILLA PRATENSIS WHOLE 6 [hp_X] in 1 mL LACTIC ACID, L- (UNII: F9S9FFU82N) (LACTIC ACID, L- - UNII:F9S9FFU82N) LACTIC ACID, L- 6 [hp_X] in 1 mL ACETYLCHOLINE CHLORIDE (UNII: AF73293C2R) (ACETYLCHOLINE - UNII:N9YNS0M02X) ACETYLCHOLINE CHLORIDE 6 [hp_X] in 1 mL MANNOSE, D- (UNII: PHA4727WTP) (MANNOSE, D- - UNII:PHA4727WTP) MANNOSE, D- 6 [hp_X] in 1 mL CONIUM MACULATUM FLOWERING TOP (UNII: Q28R5GF371) (CONIUM MACULATUM FLOWERING TOP - UNII:Q28R5GF371) CONIUM MACULATUM FLOWERING TOP 8 [hp_X] in 1 mL SUS SCROFA LYMPH (UNII: 33A7VYU29L) (SUS SCROFA LYMPH - UNII:33A7VYU29L) SUS SCROFA LYMPH 8 [hp_X] in 1 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 8 [hp_X] in 1 mL TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 10 [hp_X] in 1 mL SUS SCROFA UMBILICAL CORD (UNII: 118OYG6W3H) (SUS SCROFA UMBILICAL CORD - UNII:118OYG6W3H) SUS SCROFA UMBILICAL CORD 10 [hp_X] in 1 mL PORK LIVER (UNII: 6EC706HI7F) (PORK LIVER - UNII:6EC706HI7F) PORK LIVER 10 [hp_X] in 1 mL SUS SCROFA HYPOTHALAMUS (UNII: N6R0856Z79) (SUS SCROFA HYPOTHALAMUS - UNII:N6R0856Z79) SUS SCROFA HYPOTHALAMUS 10 [hp_X] in 1 mL SUS SCROFA BONE MARROW (UNII: VP2CN2G7Y8) (SUS SCROFA BONE MARROW - UNII:VP2CN2G7Y8) SUS SCROFA BONE MARROW 10 [hp_X] in 1 mL SUS SCROFA SPLEEN (UNII: 92AMN5J79Y) (SUS SCROFA SPLEEN - UNII:92AMN5J79Y) SUS SCROFA SPLEEN 10 [hp_X] in 1 mL CORTISONE ACETATE (UNII: 883WKN7W8X) (CORTISONE - UNII:V27W9254FZ) CORTISONE ACETATE 13 [hp_X] in 1 mL SUS SCROFA ADRENAL GLAND (UNII: 398IYQ16YV) (SUS SCROFA ADRENAL GLAND - UNII:398IYQ16YV) SUS SCROFA ADRENAL GLAND 13 [hp_X] in 1 mL MERCURIUS SOLUBILIS (UNII: 324Y4038G2) (MERCURIUS SOLUBILIS - UNII:324Y4038G2) MERCURIUS SOLUBILIS 13 [hp_X] in 1 mL SUS SCROFA THYROID (UNII: 6RV024OAUQ) (SUS SCROFA THYROID - UNII:6RV024OAUQ) SUS SCROFA THYROID 13 [hp_X] in 1 mL BARIUM CARBONATE (UNII: 6P669D8HQ8) (BARIUM CATION - UNII:V645272HLN) BARIUM CARBONATE 28 [hp_X] in 1 mL SUS SCROFA TONSIL (UNII: TB08NIC03W) (SUS SCROFA TONSIL - UNII:TB08NIC03W) SUS SCROFA TONSIL 28 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43742-1600-1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 10/29/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/29/2019 Labeler - Deseret Biologicals, Inc. (940741853) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(43742-1600) , api manufacture(43742-1600) , label(43742-1600) , pack(43742-1600)