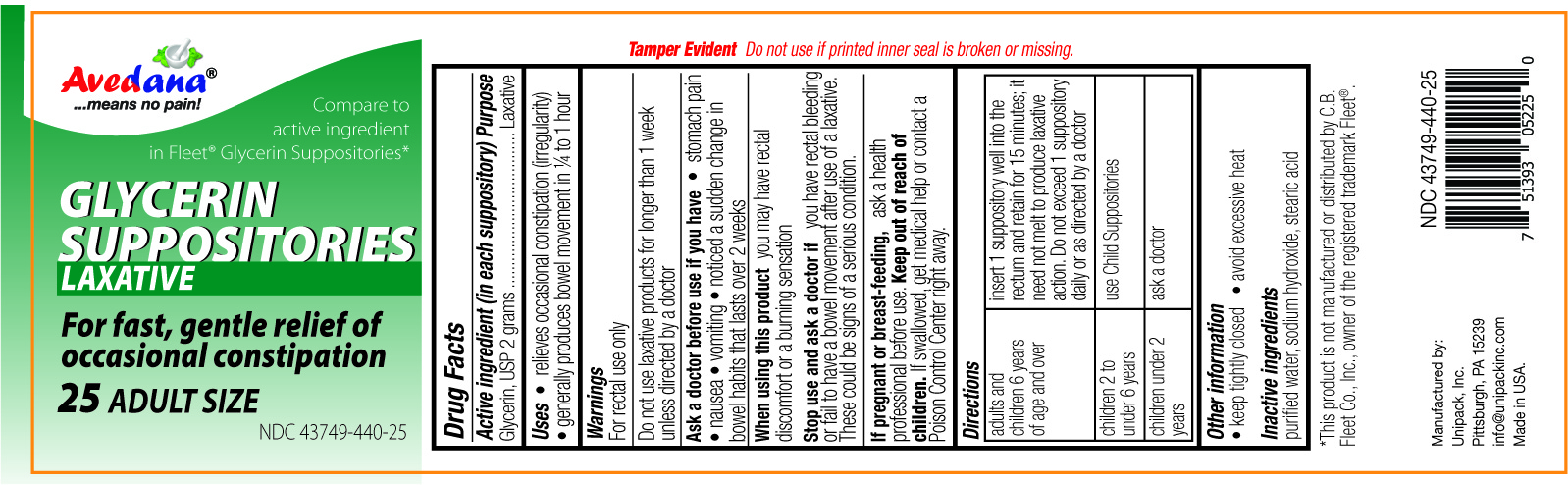
Label: AVEDANA GLYCERIN LAXATIVE- glycerin suppository
- NDC Code(s): 43749-440-25
- Packager: Unipack LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Uses
- Warnings
- Do not use
- Ask a doctor before use if you have
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Avedana ® Glycerin Suppositories | 25 Count in a Jar
-
INGREDIENTS AND APPEARANCE
AVEDANA GLYCERIN LAXATIVE
glycerin suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43749-440 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 2 g Inactive Ingredients Ingredient Name Strength STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Product Characteristics Color white (CLEAR) Score Shape BULLET Size 32mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43749-440-25 25 in 1 JAR; Type 0: Not a Combination Product 09/17/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 09/18/2014 Labeler - Unipack LLC (009248480) Registrant - Unipack LLC (116015769) Establishment Name Address ID/FEI Business Operations Unipack LLC 009248480 manufacture(43749-440)