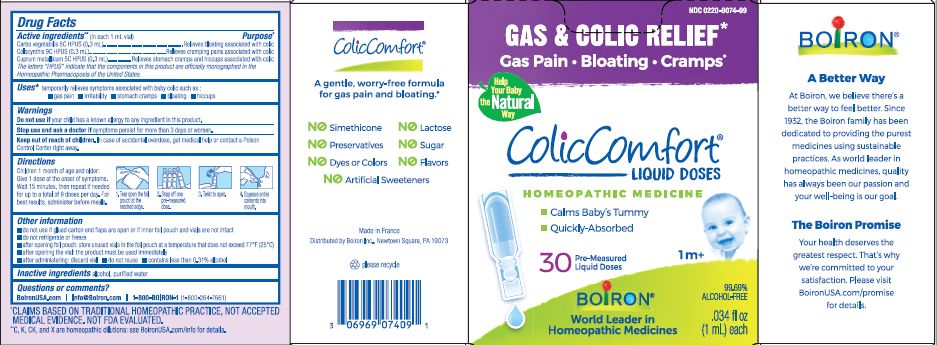
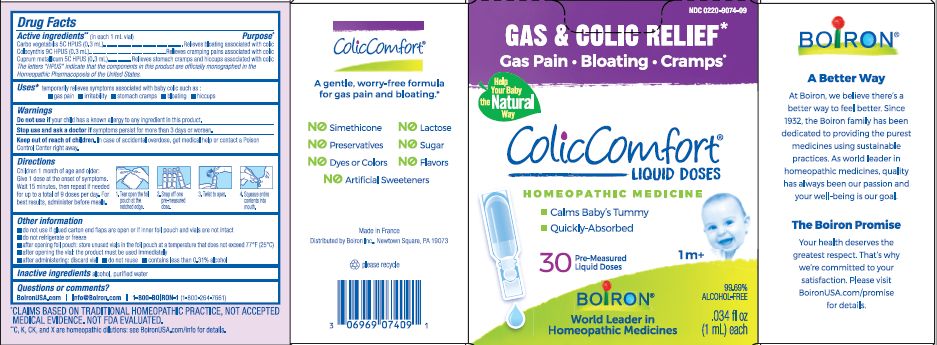
Label: COLICCOMFORT- activated charcoal, copper, citrullus colocynthis fruit liquid
- NDC Code(s): 0220-9074-09
- Packager: Boiron
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated June 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- DO NOT USE
-
DOSAGE & ADMINISTRATION
Children 1 month of age and older: Give 1 dose at the onset of symptoms, Wait 15 minutes, then repeate if needed for up to a total of 9 doses per day. For best results administer before meals.
Tear open the foil pouch at the notched edge, Snap off one pre-measured dose, twist open, squeeze entire contents into mouth.
- INACTIVE INGREDIENT
- HOW SUPPLIED
- PURPOSE
- STORAGE AND HANDLING
- QUESTIONS
- ASK DOCTOR
- WARNINGS
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
- WHEN USING
-
SPL UNCLASSIFIED SECTION
contains 0.31% alcohol
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
**C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COLICCOMFORT
activated charcoal, copper, citrullus colocynthis fruit liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0220-9074 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 5 [hp_C] COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 5 [hp_C] CITRULLUS COLOCYNTHIS FRUIT (UNII: 0E49E3V9U6) (CITRULLUS COLOCYNTHIS FRUIT - UNII:0E49E3V9U6) CITRULLUS COLOCYNTHIS FRUIT 9 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0220-9074-09 30 in 1 BOX; Type 0: Not a Combination Product 09/15/2018 10/31/2029 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/15/2018 10/31/2029 Labeler - Boiron (282560473) Registrant - Boiron (014892269) Establishment Name Address ID/FEI Business Operations Boiron 383674934 manufacture(0220-9074)