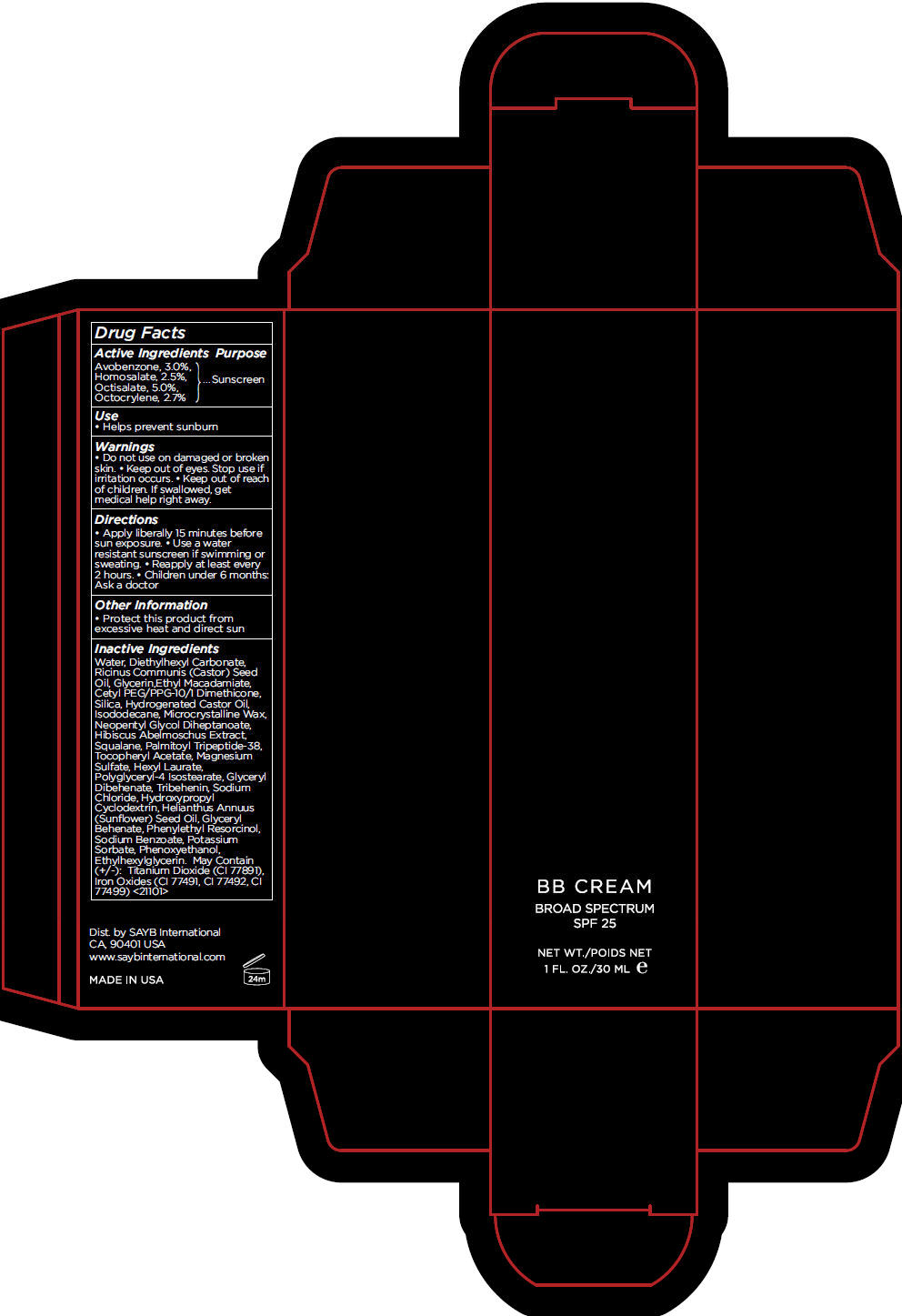
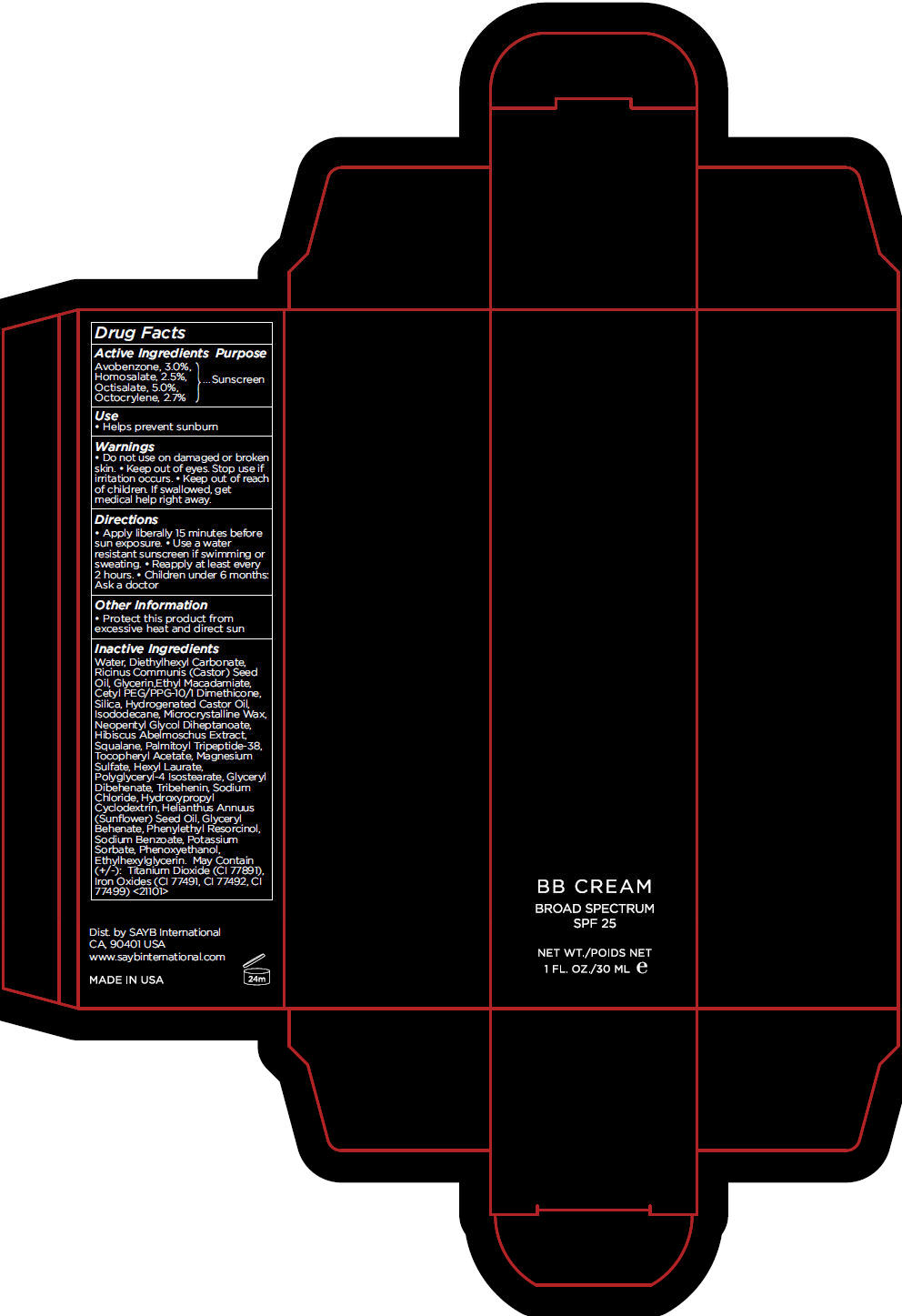
Label: BB FAIR- avobenzone, homosalate, octisalate, and octocrylene cream, augmented
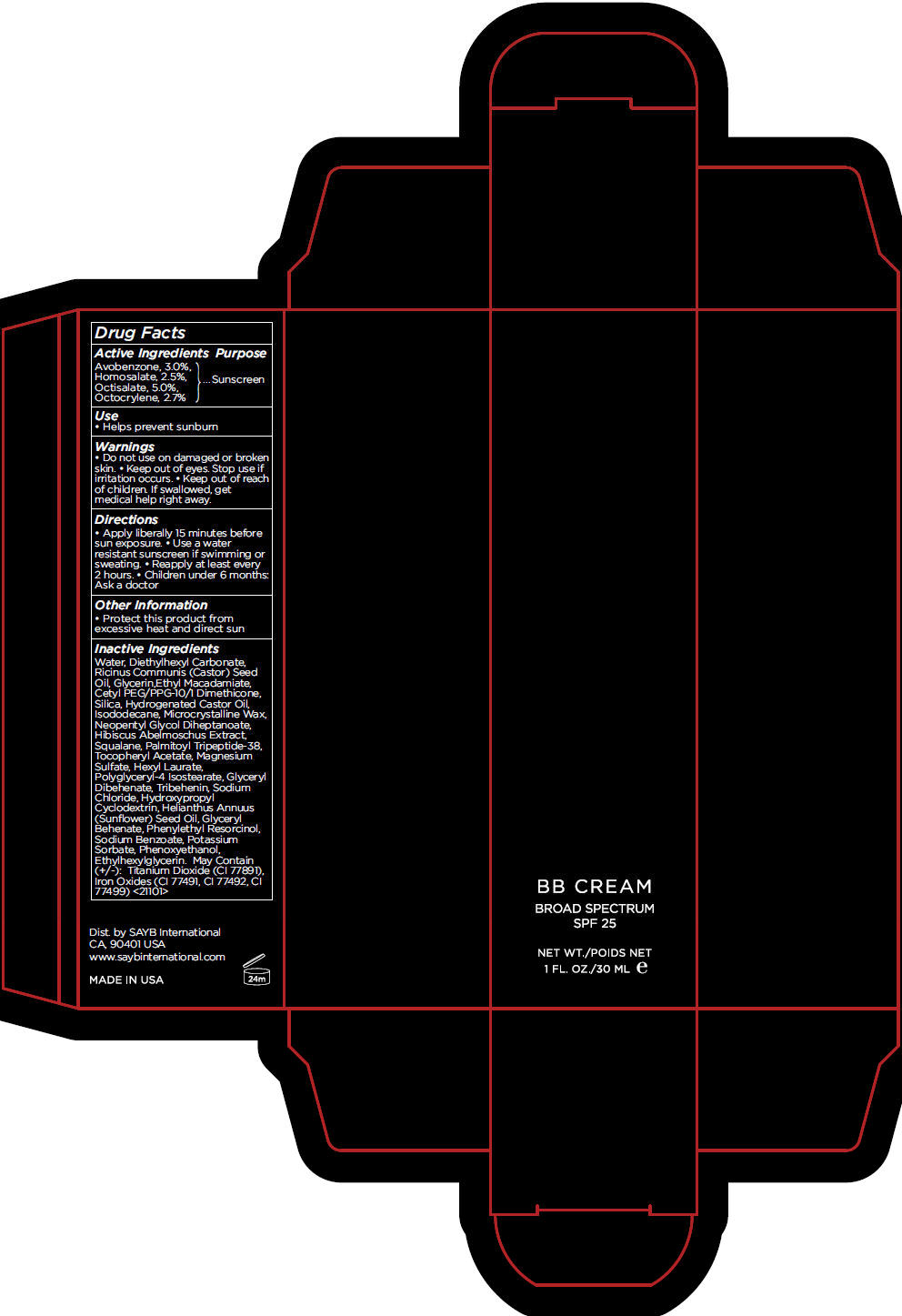
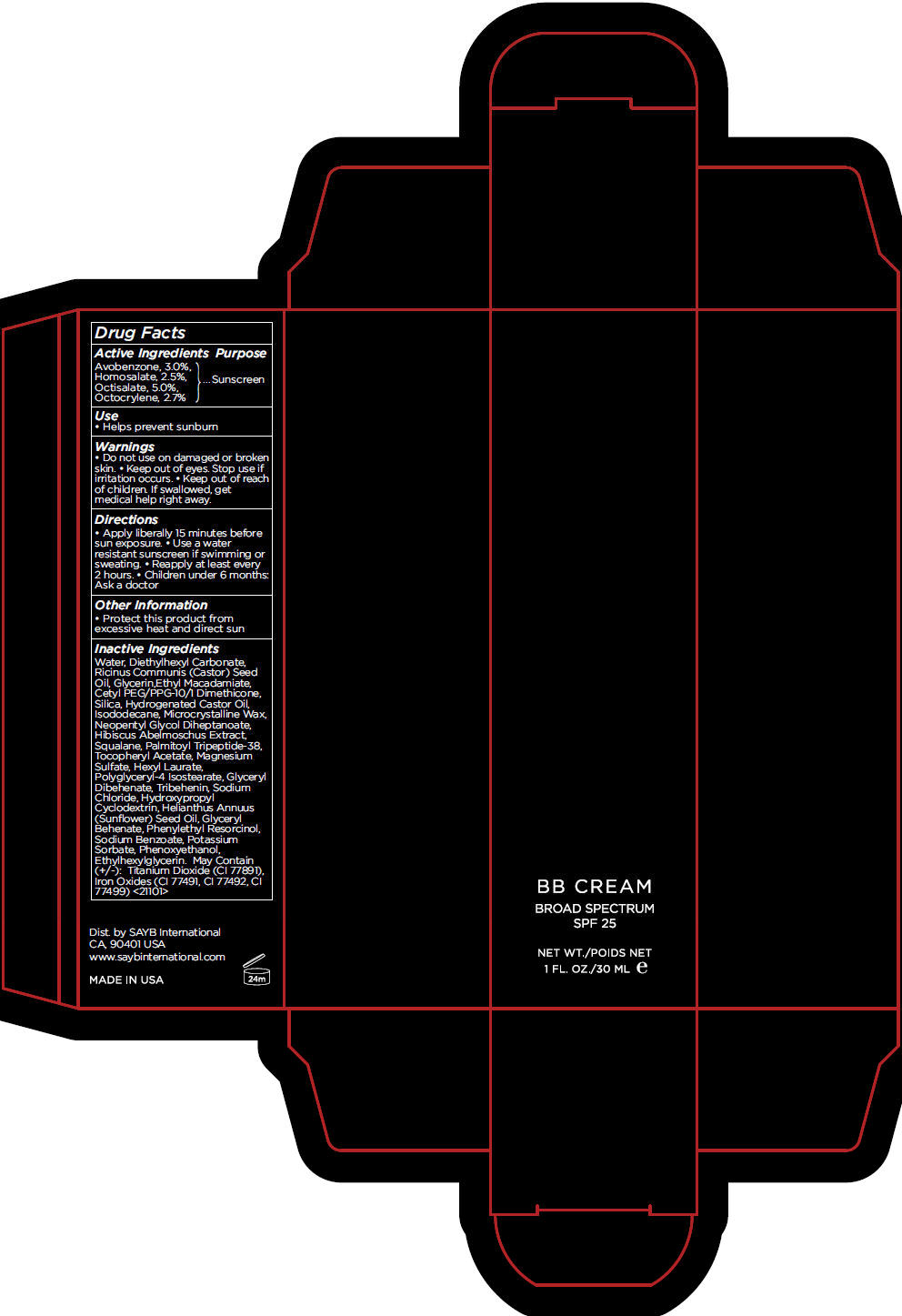
BB LIGHT- avobenzone, homosalate, octisalate, and octocrylene cream, augmented
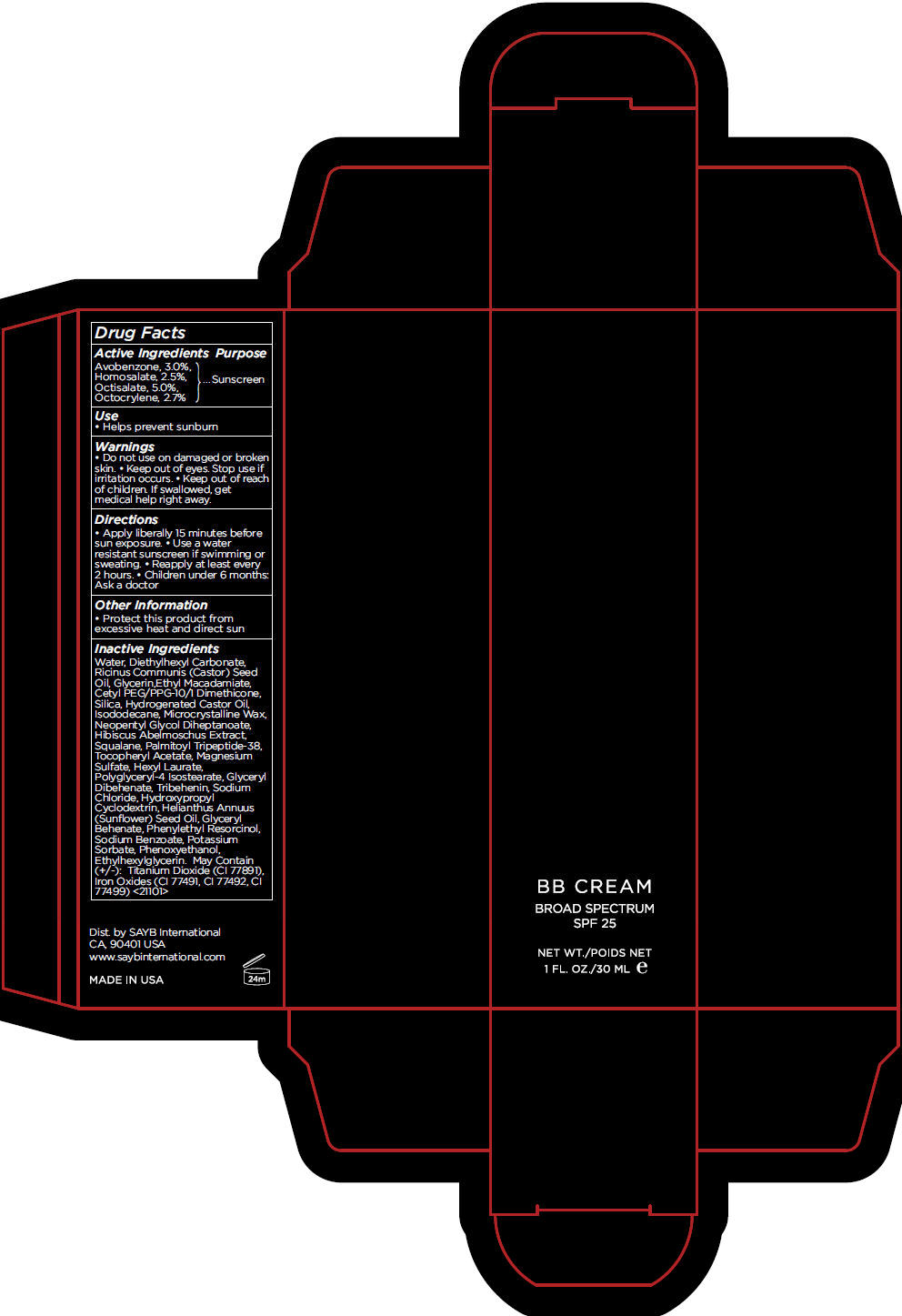
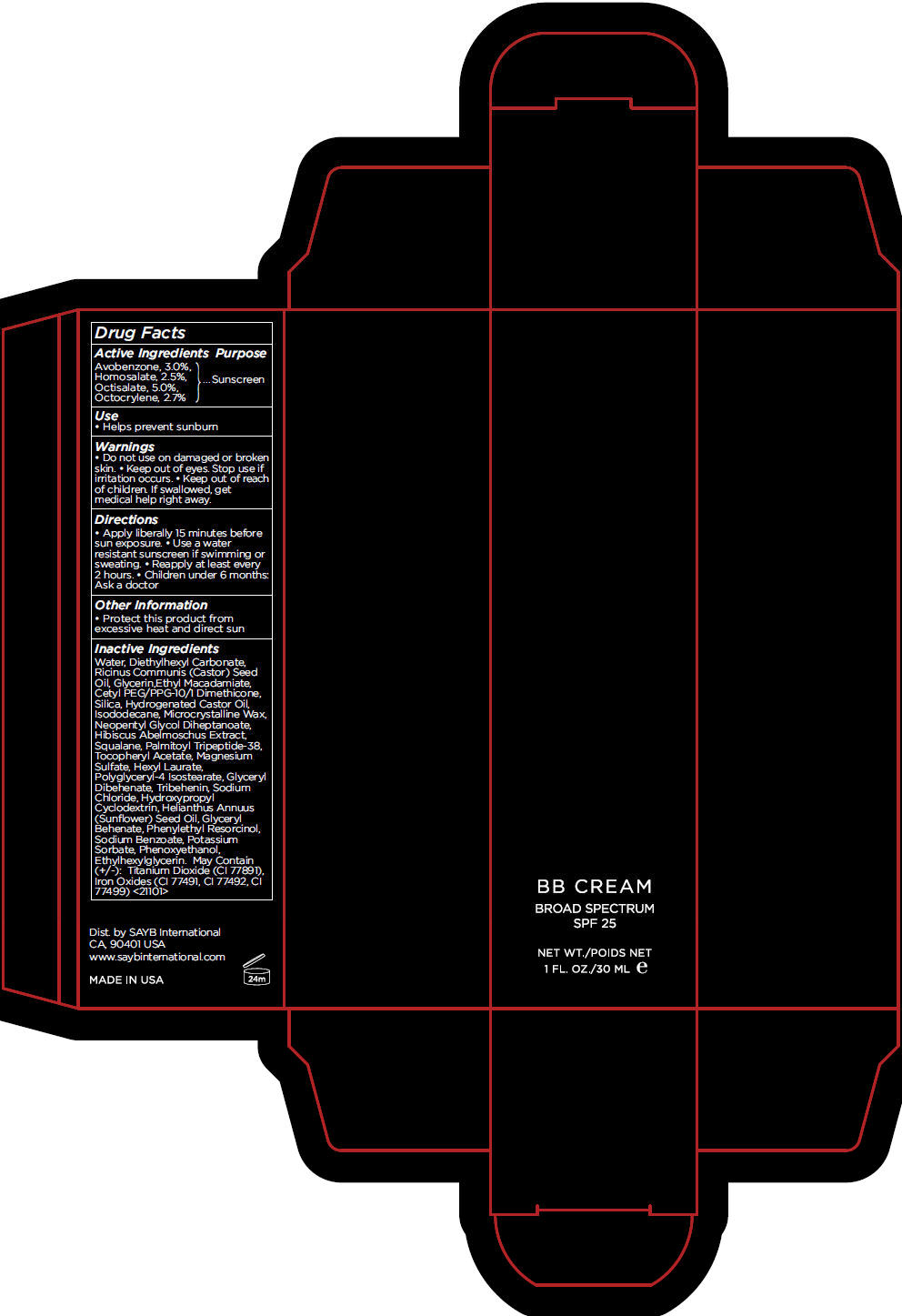
BB MEDIUM- avobenzone, homosalate, octisalate, and octocrylene cream, augmented
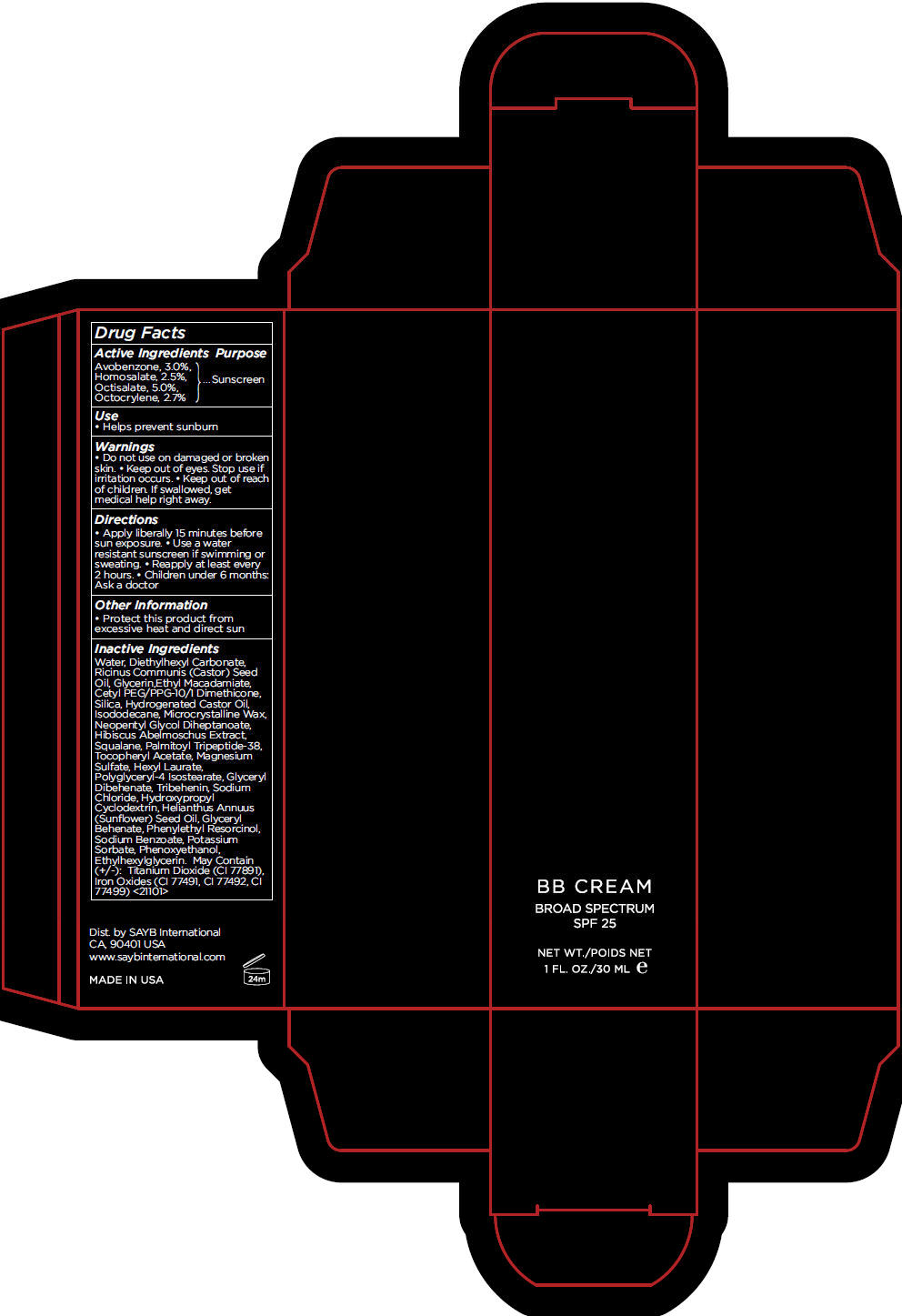
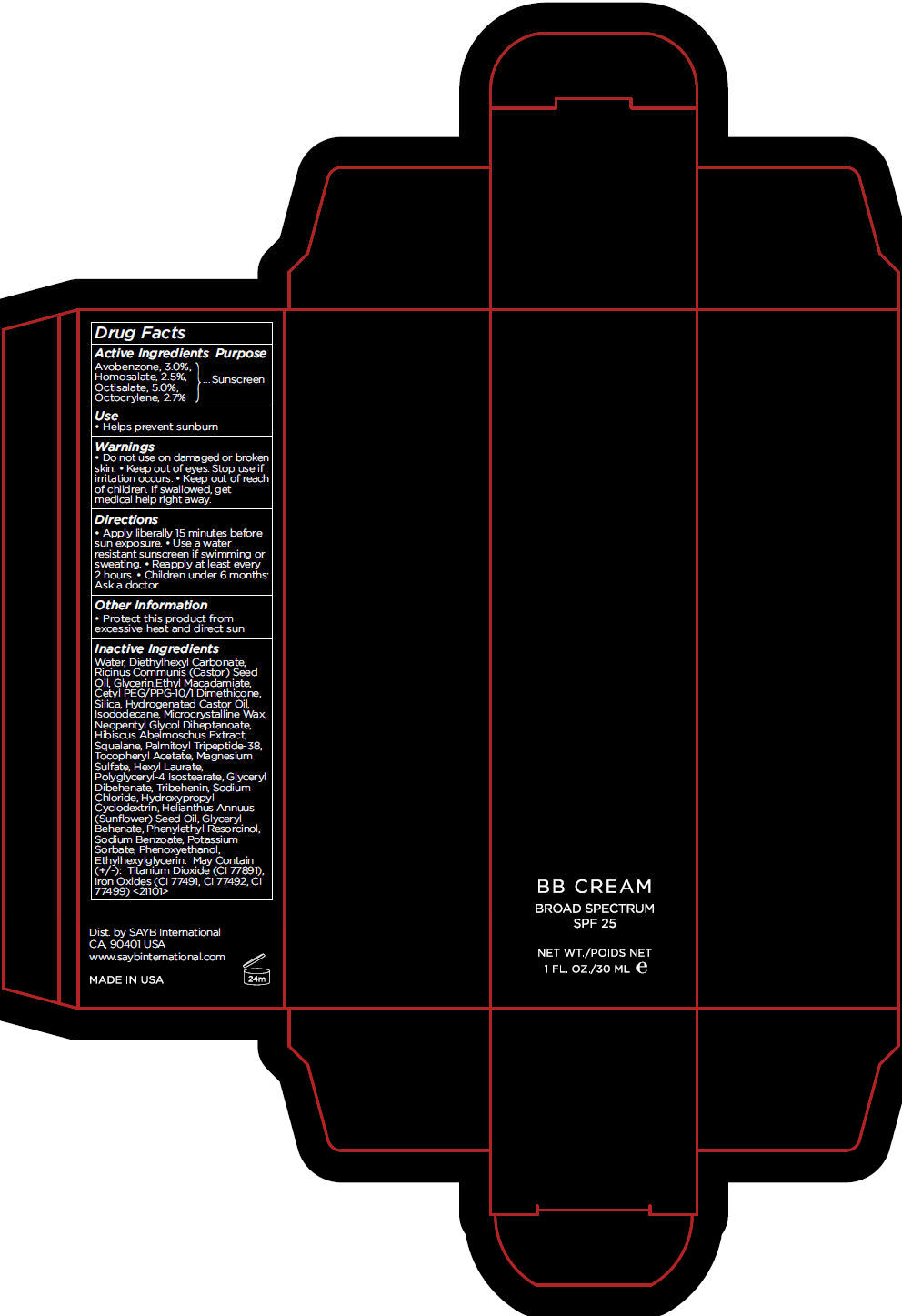
BB DARK- avobenzone, homosalate, octisalate, and octocrylene cream, augmented
-
Contains inactivated NDC Code(s)
NDC Code(s): 56001-400-30, 56001-401-30, 56001-402-30, 56001-403-30 - Packager: SAYB International
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 29, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Use
- Warnings
- Directions
- Other Information
-
Inactive Ingredients
Water, Diethylhexyl Carbonate, Ricinus Communis (Castor) Seed Oil, Glycerin,Ethyl Macadamiate, Cetyl PEG/PPG-10/1 Dimethicone, Silica, Hydrogenated Castor Oil, Isododecane, Microcrystalline Wax, Neopentyl Glycol Diheptanoate, Hibiscus Abelmoschus Extract, Squalane, Palmitoyl Tripeptide-38, Tocopheryl Acetate, Magnesium Sulfate, Hexyl Laurate, Polyglyceryl-4 Isostearate, Glyceryl Dibehenate, Tribehenin, Sodium Chloride, Hydroxypropyl Cyclodextrin, Helianthus Annuus (Sunflower) Seed Oil, Glyceryl Behenate, Phenylethyl Resorcinol, Sodium Benzoate, Potassium Sorbate, Phenoxyethanol, Ethylhexylglycerin. May Contain (+/-): Titanium Dioxide (CI 77891), Iron Oxides (CI 77491, CI 77492, CI 77499) <21101>
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - FAIR
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - LIGHT
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - MEDIUM
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton - DARK
-
INGREDIENTS AND APPEARANCE
BB FAIR
avobenzone, homosalate, octisalate, and octocrylene cream, augmentedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:56001-400 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 30 mg in 1 mL Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 25 mg in 1 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 50 mg in 1 mL Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 27 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Diethylhexyl Carbonate (UNII: YCD50O0Z6L) Castor Oil (UNII: D5340Y2I9G) Glycerin (UNII: PDC6A3C0OX) Ethyl Macadamiate (UNII: ANA2NCS6V1) Cetyl PEG/PPG-10/1 Dimethicone (HLB 2) (UNII: V2W71V8T0X) Silicon Dioxide (UNII: ETJ7Z6XBU4) Hydrogenated Castor Oil (UNII: ZF94AP8MEY) Isododecane (UNII: A8289P68Y2) Microcrystalline Wax (UNII: XOF597Q3KY) Neopentyl Glycol Diheptanoate (UNII: 5LKW3C543X) Abelmoschus Moschatus Seed (UNII: UN2QZ55I88) Squalane (UNII: GW89575KF9) Palmitoyl Lysyldioxymethionyllysine (UNII: T7A529FB8O) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Magnesium Sulfate (UNII: DE08037SAB) Hexyl Laurate (UNII: 4CG9F9W01Q) Polyglyceryl-4 Isostearate (UNII: 820DPX33S7) Glyceryl Dibehenate (UNII: R8WTH25YS2) Tribehenin (UNII: 8OC9U7TQZ0) Sodium Chloride (UNII: 451W47IQ8X) Hydroxypropyl .Beta.-Cyclodextrin (UNII: 1I96OHX6EK) Sunflower Oil (UNII: 3W1JG795YI) Phenylethyl Resorcinol (UNII: G37UFG162O) Sodium Benzoate (UNII: OJ245FE5EU) Potassium Sorbate (UNII: 1VPU26JZZ4) Phenoxyethanol (UNII: HIE492ZZ3T) Ethylhexylglycerin (UNII: 147D247K3P) Titanium Dioxide (UNII: 15FIX9V2JP) Ferric Oxide Yellow (UNII: EX438O2MRT) Ferric Oxide Red (UNII: 1K09F3G675) Ferrosoferric Oxide (UNII: XM0M87F357) Product Characteristics Color YELLOW (SKIN COLOR) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:56001-400-30 1 in 1 CARTON 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 03/15/2013 BB LIGHT
avobenzone, homosalate, octisalate, and octocrylene cream, augmentedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:56001-401 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 30 mg in 1 mL Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 25 mg in 1 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 50 mg in 1 mL Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 27 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Diethylhexyl Carbonate (UNII: YCD50O0Z6L) Castor Oil (UNII: D5340Y2I9G) Glycerin (UNII: PDC6A3C0OX) Ethyl Macadamiate (UNII: ANA2NCS6V1) Cetyl PEG/PPG-10/1 Dimethicone (HLB 2) (UNII: V2W71V8T0X) Silicon Dioxide (UNII: ETJ7Z6XBU4) Hydrogenated Castor Oil (UNII: ZF94AP8MEY) Isododecane (UNII: A8289P68Y2) Microcrystalline Wax (UNII: XOF597Q3KY) Neopentyl Glycol Diheptanoate (UNII: 5LKW3C543X) Abelmoschus Moschatus Seed (UNII: UN2QZ55I88) Squalane (UNII: GW89575KF9) Palmitoyl Lysyldioxymethionyllysine (UNII: T7A529FB8O) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Magnesium Sulfate (UNII: DE08037SAB) Hexyl Laurate (UNII: 4CG9F9W01Q) Polyglyceryl-4 Isostearate (UNII: 820DPX33S7) Glyceryl Dibehenate (UNII: R8WTH25YS2) Tribehenin (UNII: 8OC9U7TQZ0) Sodium Chloride (UNII: 451W47IQ8X) Hydroxypropyl .Beta.-Cyclodextrin (UNII: 1I96OHX6EK) Sunflower Oil (UNII: 3W1JG795YI) Phenylethyl Resorcinol (UNII: G37UFG162O) Sodium Benzoate (UNII: OJ245FE5EU) Potassium Sorbate (UNII: 1VPU26JZZ4) Phenoxyethanol (UNII: HIE492ZZ3T) Ethylhexylglycerin (UNII: 147D247K3P) Titanium Dioxide (UNII: 15FIX9V2JP) Ferric Oxide Yellow (UNII: EX438O2MRT) Ferric Oxide Red (UNII: 1K09F3G675) Ferrosoferric Oxide (UNII: XM0M87F357) Product Characteristics Color YELLOW (SKIN COLOR) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:56001-401-30 1 in 1 CARTON 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 03/15/2013 BB MEDIUM
avobenzone, homosalate, octisalate, and octocrylene cream, augmentedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:56001-402 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 30 mg in 1 mL Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 25 mg in 1 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 50 mg in 1 mL Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 27 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Diethylhexyl Carbonate (UNII: YCD50O0Z6L) Castor Oil (UNII: D5340Y2I9G) Glycerin (UNII: PDC6A3C0OX) Ethyl Macadamiate (UNII: ANA2NCS6V1) Cetyl PEG/PPG-10/1 Dimethicone (HLB 2) (UNII: V2W71V8T0X) Silicon Dioxide (UNII: ETJ7Z6XBU4) Hydrogenated Castor Oil (UNII: ZF94AP8MEY) Isododecane (UNII: A8289P68Y2) Microcrystalline Wax (UNII: XOF597Q3KY) Neopentyl Glycol Diheptanoate (UNII: 5LKW3C543X) Abelmoschus Moschatus Seed (UNII: UN2QZ55I88) Squalane (UNII: GW89575KF9) Palmitoyl Lysyldioxymethionyllysine (UNII: T7A529FB8O) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Magnesium Sulfate (UNII: DE08037SAB) Hexyl Laurate (UNII: 4CG9F9W01Q) Polyglyceryl-4 Isostearate (UNII: 820DPX33S7) Glyceryl Dibehenate (UNII: R8WTH25YS2) Tribehenin (UNII: 8OC9U7TQZ0) Sodium Chloride (UNII: 451W47IQ8X) Hydroxypropyl .Beta.-Cyclodextrin (UNII: 1I96OHX6EK) Sunflower Oil (UNII: 3W1JG795YI) Phenylethyl Resorcinol (UNII: G37UFG162O) Sodium Benzoate (UNII: OJ245FE5EU) Potassium Sorbate (UNII: 1VPU26JZZ4) Phenoxyethanol (UNII: HIE492ZZ3T) Ethylhexylglycerin (UNII: 147D247K3P) Titanium Dioxide (UNII: 15FIX9V2JP) Ferric Oxide Yellow (UNII: EX438O2MRT) Ferric Oxide Red (UNII: 1K09F3G675) Ferrosoferric Oxide (UNII: XM0M87F357) Product Characteristics Color YELLOW (SKIN COLOR) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:56001-402-30 1 in 1 CARTON 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 03/15/2013 BB DARK
avobenzone, homosalate, octisalate, and octocrylene cream, augmentedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:56001-403 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 30 mg in 1 mL Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 25 mg in 1 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 50 mg in 1 mL Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 27 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Diethylhexyl Carbonate (UNII: YCD50O0Z6L) Castor Oil (UNII: D5340Y2I9G) Glycerin (UNII: PDC6A3C0OX) Ethyl Macadamiate (UNII: ANA2NCS6V1) Cetyl PEG/PPG-10/1 Dimethicone (HLB 2) (UNII: V2W71V8T0X) Silicon Dioxide (UNII: ETJ7Z6XBU4) Hydrogenated Castor Oil (UNII: ZF94AP8MEY) Isododecane (UNII: A8289P68Y2) Microcrystalline Wax (UNII: XOF597Q3KY) Neopentyl Glycol Diheptanoate (UNII: 5LKW3C543X) Abelmoschus Moschatus Seed (UNII: UN2QZ55I88) Squalane (UNII: GW89575KF9) Palmitoyl Lysyldioxymethionyllysine (UNII: T7A529FB8O) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Magnesium Sulfate (UNII: DE08037SAB) Hexyl Laurate (UNII: 4CG9F9W01Q) Polyglyceryl-4 Isostearate (UNII: 820DPX33S7) Glyceryl Dibehenate (UNII: R8WTH25YS2) Tribehenin (UNII: 8OC9U7TQZ0) Sodium Chloride (UNII: 451W47IQ8X) Hydroxypropyl .Beta.-Cyclodextrin (UNII: 1I96OHX6EK) Sunflower Oil (UNII: 3W1JG795YI) Phenylethyl Resorcinol (UNII: G37UFG162O) Sodium Benzoate (UNII: OJ245FE5EU) Potassium Sorbate (UNII: 1VPU26JZZ4) Phenoxyethanol (UNII: HIE492ZZ3T) Ethylhexylglycerin (UNII: 147D247K3P) Titanium Dioxide (UNII: 15FIX9V2JP) Ferric Oxide Yellow (UNII: EX438O2MRT) Ferric Oxide Red (UNII: 1K09F3G675) Ferrosoferric Oxide (UNII: XM0M87F357) Product Characteristics Color YELLOW (SKIN COLOR) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:56001-403-30 1 in 1 CARTON 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 03/15/2013 Labeler - SAYB International (958886942)