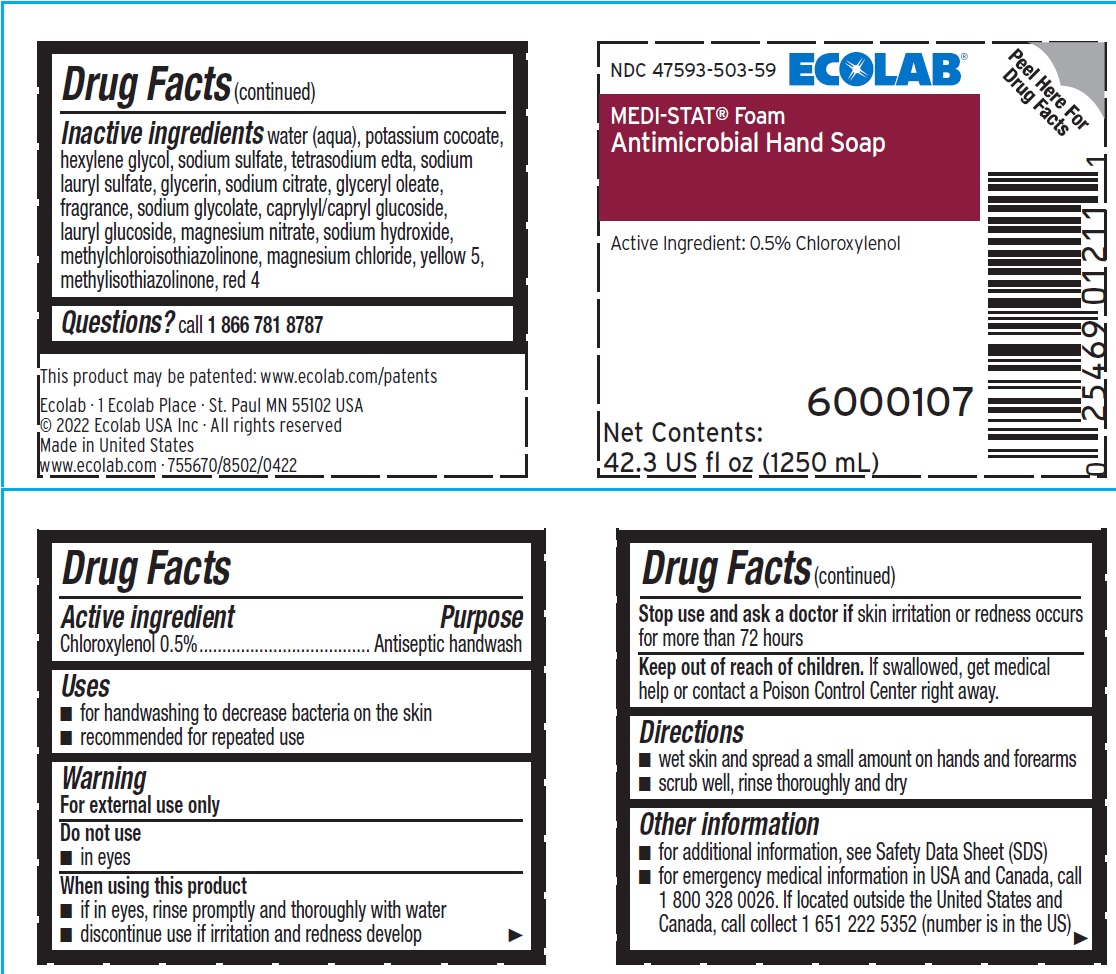
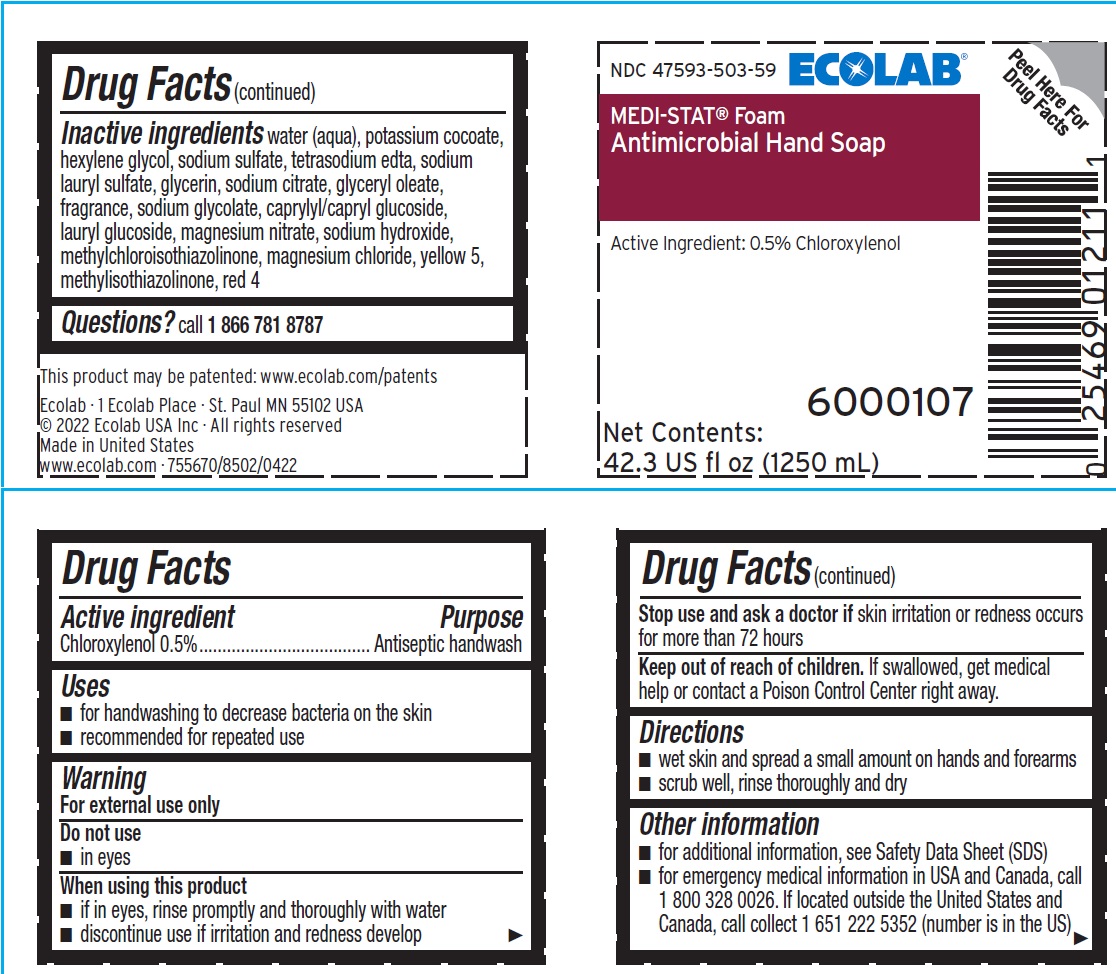
Label: ECOLAB INC.- chloroxylenol liquid
- NDC Code(s): 47593-503-41, 47593-503-59
- Packager: Ecolab Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
INACTIVE INGREDIENT
Inactive ingredients water (aqua), potassium cocoate, hexylene glycol, sodium sulfate, tetrasodium edta, sodium lauryl sulfate, glycerin, sodium citrate, glyceryl oleate, fragrance, sodium glycolate, caprylyl/capryl glucoside, lauryl glucoside, magnesium nitrate, sodium hydroxide, methylchloroisothiazolinone, magnesium chloride, yellow 5, methylisothiazolinone, red 4
- QUESTIONS
- Principal display panel and representative label
-
INGREDIENTS AND APPEARANCE
ECOLAB INC.
chloroxylenol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47593-503 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POTASSIUM COCOATE (UNII: F8U72V8ZXP) HEXYLENE GLYCOL (UNII: KEH0A3F75J) SODIUM SULFATE (UNII: 0YPR65R21J) EDETATE SODIUM (UNII: MP1J8420LU) SODIUM LAURYL SULFATE (UNII: 368GB5141J) GLYCERIN (UNII: PDC6A3C0OX) SODIUM CITRATE (UNII: 1Q73Q2JULR) GLYCERYL OLEATE (UNII: 4PC054V79P) SODIUM GLYCOLATE (UNII: B75E535IMI) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) MAGNESIUM NITRATE (UNII: 77CBG3UN78) SODIUM HYDROXIDE (UNII: 55X04QC32I) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) FD&C RED NO. 4 (UNII: X3W0AM1JLX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47593-503-41 750 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/27/2013 2 NDC:47593-503-59 1250 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/27/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 10/27/2013 Labeler - Ecolab Inc. (006154611)