Label: TETRACAINE HYDROCHLORIDE solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 76519-1118-5 - Packager: H.J. Harkins Company, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 24, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Tetracaine Hydrochloride is a sterile aqueous topical anesthetic ophthalmic solution. The active ingredient is represented by the chemical structure:
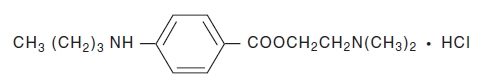
C15H24N2O2·HCI
Mol. wt. 300.83Benzoic acid, 4-[butylamino]-, 2-[dimethylamino]ethyl ester, monohydrochloride.
EACH mL CONTAINS: ACTIVE: Tetracaine Hydrochloride 5 mg (0.5%); INACTIVES: Boric Acid, Potassium Chloride, Edetate Disodium and Purified Water. Sodium Hydroxide and/or Hydrochloric Acid may be added to adjust pH (3.7 - 6.0). PRESERVATIVE ADDED: Chlorobutanol 0.4%.
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
FOR TOPICAL USE ONLY—NOT FOR INJECTION. To prevent contaminating the dropper tip and solution, care should be taken not to touch the eyelids or surrounding area with the dropper tip. Patient should be advised not to touch or rub the eye(s) until the effect of the anesthetic has worn off.
Information to the Patient:
After instillation of this product, the surface of the eye is insensitive and can be scratched without feeling it. Do not rub eye. Do not instill this product repeatedly because severe eye damage may occur.
DO NOT USE IF SOLUTION CONTAINS CRYSTALS, OR IS CLOUDY OR DISCOLORED.
-
ADVERSE REACTIONS
Transient symptoms (signs) such as stinging, burning and conjunctival redness may occur. A rare, severe, immediate allergic cornea reaction has been reported, characterized by acute diffuse epithelial keratitis with filament formation and/or sloughing of large areas of necrotic epithelium, diffuse stromal edema, descemetitis and iritis.
-
DOSAGE & ADMINISTRATION
For tonometry and other procedures of short duration, instill one or two drops just prior to evaluation. For minor surgical procedures such as foreign body or suture removal, administer one to two drops every five to ten minutes for one to three instillations. For prolonged anesthesia as in cataract extraction, instill one or two drops in the eye(s) every five to ten minutes for three to five doses.

- HOW SUPPLIED
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TETRACAINE HYDROCHLORIDE
tetracaine hydrochloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76519-1118 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TETRACAINE HYDROCHLORIDE (UNII: 5NF5D4OPCI) (TETRACAINE - UNII:0619F35CGV) TETRACAINE HYDROCHLORIDE 5 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76519-1118-5 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 01/28/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/28/2016 Labeler - H.J. Harkins Company, Inc. (147681894) Establishment Name Address ID/FEI Business Operations H.J. Harkins Company, Inc. 147681894 manufacture(76519-1118) , relabel(76519-1118) , repack(76519-1118)


