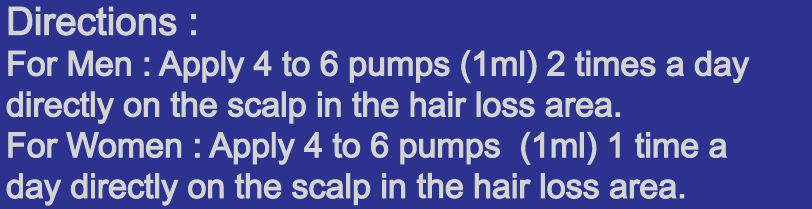Label: GROMEDI 5X- minoxidil 5% spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 71331-104-04 - Packager: Orange Lab
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 1, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Ask a doctor
- DO NOT USE IF
- Maybe be harmful if used when pregnant or breast-feeding.
- When using this product
- Inactive ingredient
- Directions
- Do not use on children
- Directions for dosage / frequency
- Dosage and Administration
- Warnings section
- Indications and usage
- Keep out of reach of children
- Purpose
- Front display panel
-
INGREDIENTS AND APPEARANCE
GROMEDI 5X
minoxidil 5% sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71331-104 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 5 g in 100 g Inactive Ingredients Ingredient Name Strength APIGENIN (UNII: 7V515PI7F6) BIOTINOYL TRIPEPTIDE-1 (UNII: O6380721VA) SAW PALMETTO (UNII: J7WWH9M8QS) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) OLEANOLIC ACID (UNII: 6SMK8R7TGJ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) URTICA DIOICA ROOT (UNII: J8HE8A6E5T) PPG-26-BUTETH-26 (UNII: 2II1K6TZ4P) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BENZYL ALCOHOL (UNII: LKG8494WBH) BENZOIC ACID (UNII: 8SKN0B0MIM) EDETATE SODIUM (UNII: MP1J8420LU) PANTHENOL (UNII: WV9CM0O67Z) BIOTIN (UNII: 6SO6U10H04) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) GREEN TEA LEAF (UNII: W2ZU1RY8B0) BEE PROPOLIS EXTRACT (UNII: 6Y8XYV2NOF) PAULLINIA CUPANA SEED (UNII: C21GE7524R) ASIAN GINSENG (UNII: CUQ3A77YXI) MENTHOL (UNII: L7T10EIP3A) CETRIMONIUM BROMIDE (UNII: L64N7M9BWR) ALOE VERA LEAF (UNII: ZY81Z83H0X) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) DEHYDROACETIC ACID (UNII: 2KAG279R6R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71331-104-04 118 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 08/23/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/22/2018 Labeler - Orange Lab (004862271) Registrant - Orange Lab (004862271) Establishment Name Address ID/FEI Business Operations Orange Lab 004862271 manufacture(71331-104)