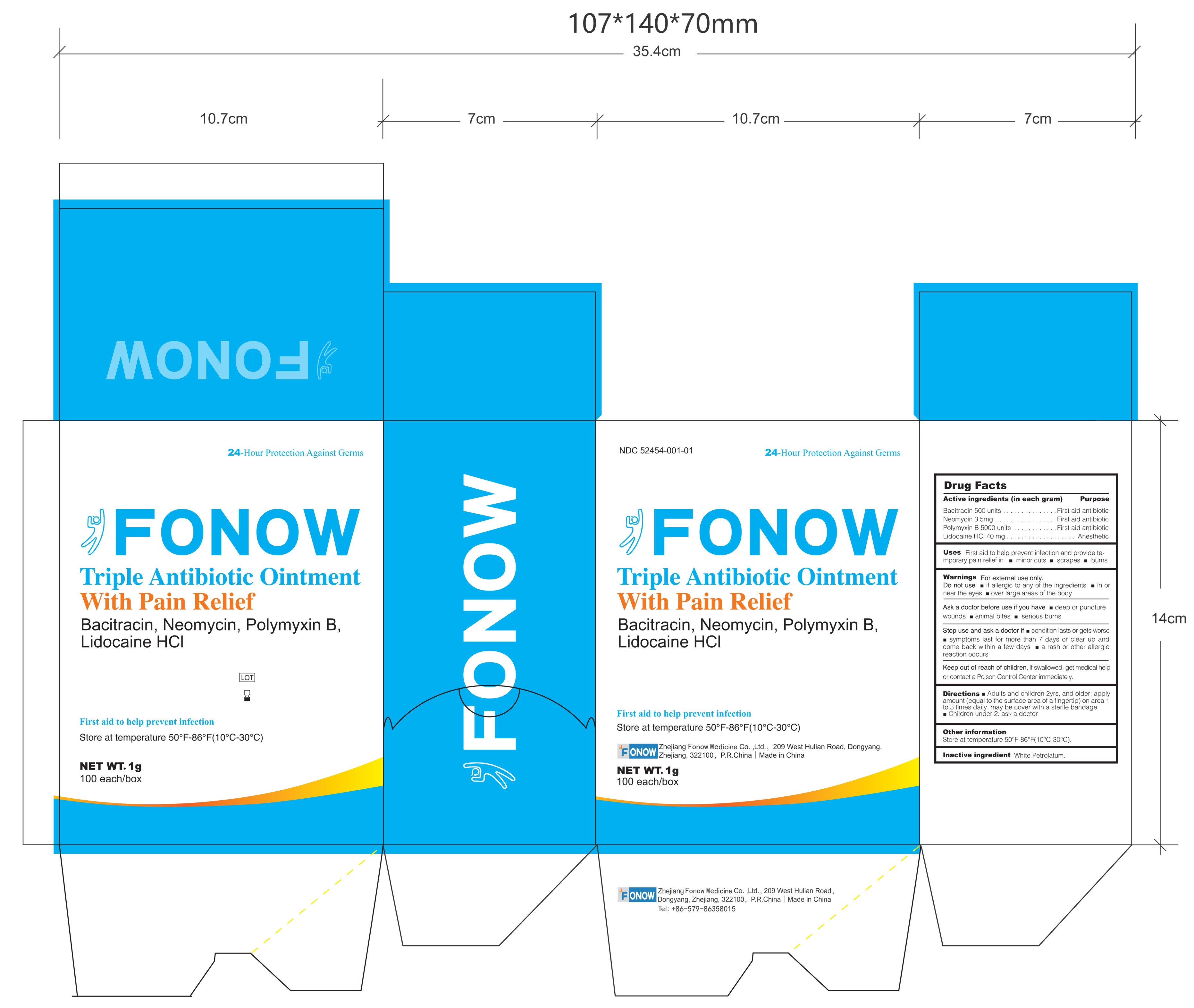
Label: FONOW- bacitracin, neomycin, polymyxin b, lidocaine hcl ointment
- NDC Code(s): 52454-001-01, 52454-001-02, 52454-001-03
- Packager: Zhejiang Fonow Medicine Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients (in each gram)
- Purpose
- INDICATIONS & USAGE
- Warnings
- Do not use
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Inactive ingredient
-
PRINCIPAL DISPLAY PANEL
Drug Facts
Active ingredients (in each gram) Purpose
Bacitracin 500 units .....................................................First aid antibiotic
Neomycin 3.5mg ............................................................First aid antibiotic
Polymyxin B 5000 units..................................................First aid antibiotic
Lidocaine Hcl 40 mg........................ .... .... ... ........................ AnestheticUses Fist aid to help prevent infection and provide temporary pain relief in minor cuts scrapes burns
Warnings
For external use only.
Do not use
if allergic to any of the ingredients
in or near the eyes
over large areas of the body
Ask a doctor before use if you have
deep or puncture wounds
animal bites
serious burns
Stop use and ask a doctor if
condition lasts or gets worse
symptoms last for more than 7 days or clear up and come back within a few days
a rash or other allergic reaction occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
Adults and children 2yrs. and older: apply amount (equal to the surface area of a fingertip) on area 1 to 3 times daily. may be cover with a sterile bandage
Children under 2: ask a doctor
Other information Store at temperature 50°-86℉(10°-30℃)
Inactive ingredient White Petrolatum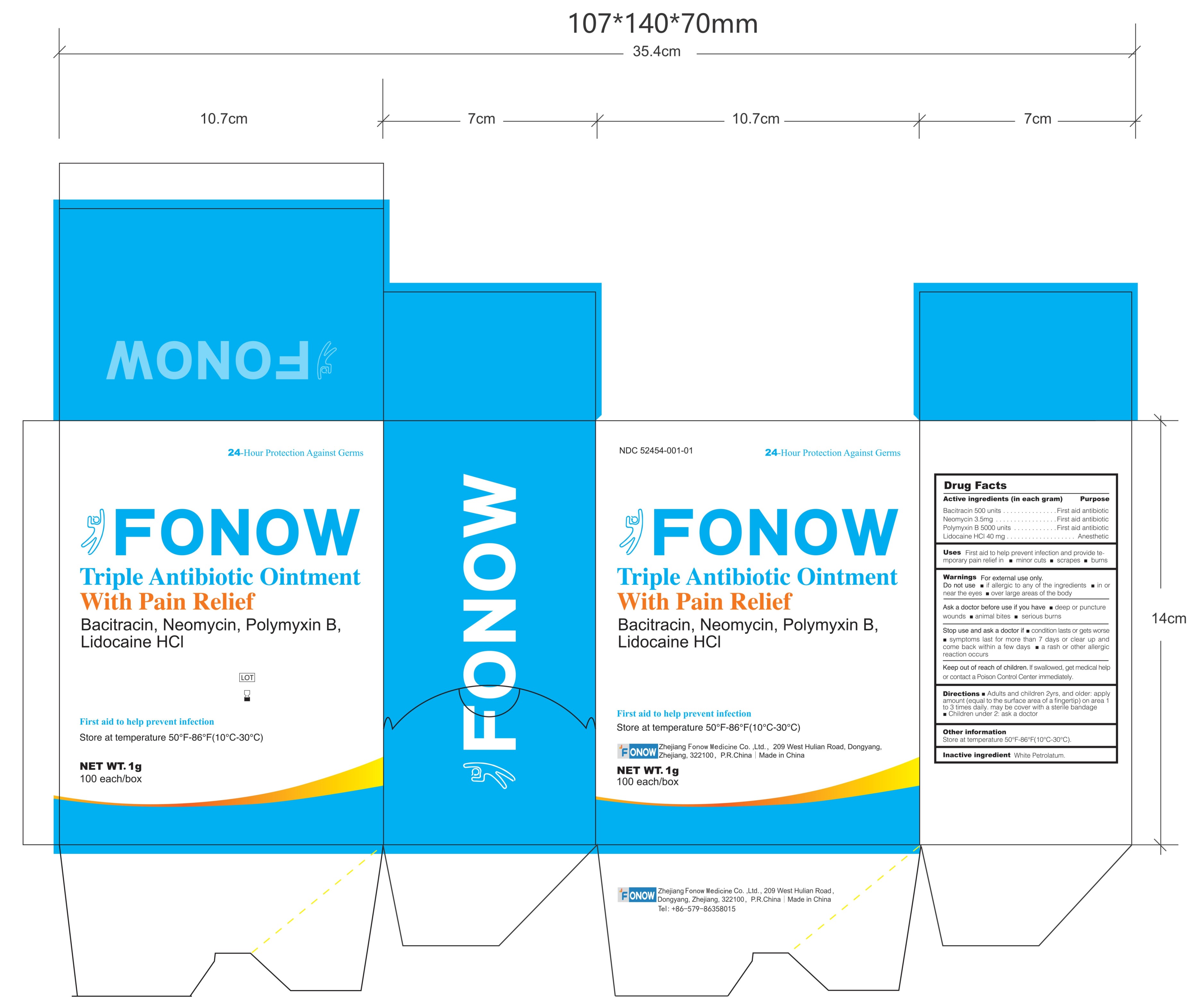


-
INGREDIENTS AND APPEARANCE
FONOW
bacitracin, neomycin, polymyxin b, lidocaine hcl ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52454-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN (UNII: 58H6RWO52I) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 500 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 40 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) 945 mg in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52454-001-01 100 in 1 BOX 01/01/2018 1 1 g in 1 POUCH; Type 0: Not a Combination Product 2 NDC:52454-001-02 1 in 1 BOX 01/01/2018 2 15 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:52454-001-03 25 in 1 BOX 01/01/2018 3 1 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 01/01/2018 Labeler - Zhejiang Fonow Medicine Co., Ltd. (420094818) Establishment Name Address ID/FEI Business Operations Zhejiang Fonow Medicine Co., Ltd. 420094818 manufacture(52454-001)