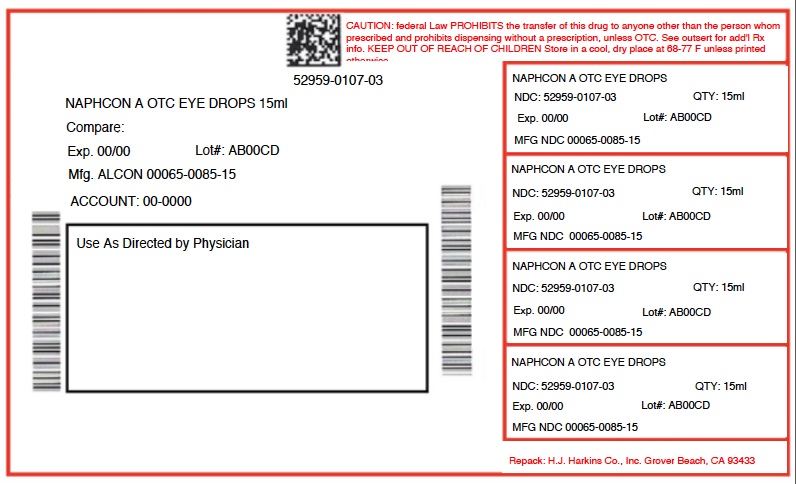
Label: NAPHCON A- naphazoline hydrochloride and pheniramine maleate solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 52959-107-03 - Packager: H.J. Harkins Company, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 21, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- PURPOSE
- ACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NAPHCON A
naphazoline hydrochloride and pheniramine maleate solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52959-107 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAPHAZOLINE HYDROCHLORIDE (UNII: MZ1131787D) (NAPHAZOLINE - UNII:H231GF11BV) NAPHAZOLINE HYDROCHLORIDE 0.25 mg in 1 mL PHENIRAMINE MALEATE (UNII: NYW905655B) (PHENIRAMINE - UNII:134FM9ZZ6M) PHENIRAMINE MALEATE 3 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) WATER (UNII: 059QF0KO0R) SODIUM BORATE (UNII: 91MBZ8H3QO) SODIUM CHLORIDE (UNII: 451W47IQ8X) BORIC ACID (UNII: R57ZHV85D4) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52959-107-03 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/26/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020226 01/26/2016 Labeler - H.J. Harkins Company, Inc. (147681894) Establishment Name Address ID/FEI Business Operations H.J. Harkins Company, Inc. 147681894 relabel(52959-107) , repack(52959-107) , manufacture(52959-107)