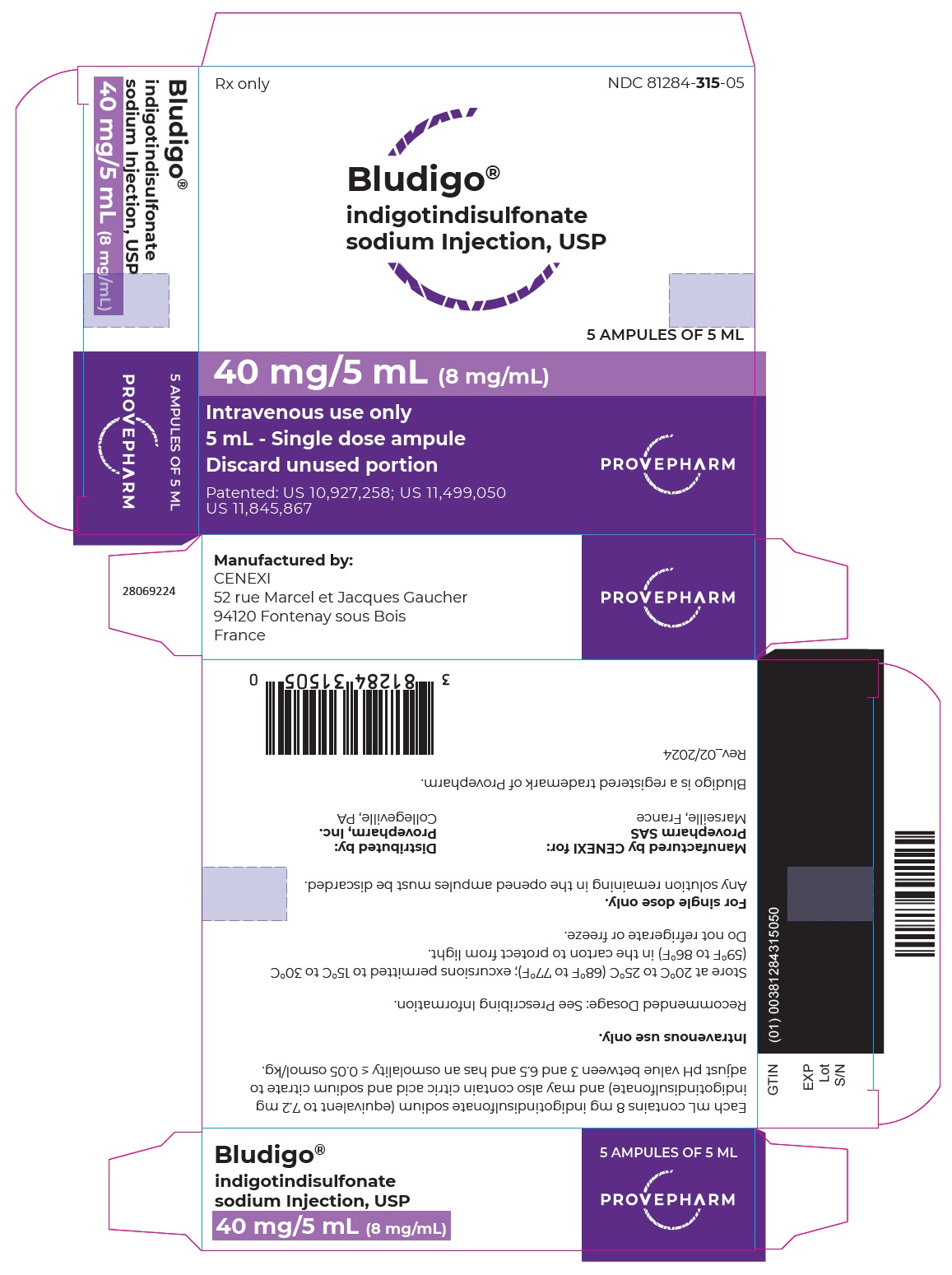
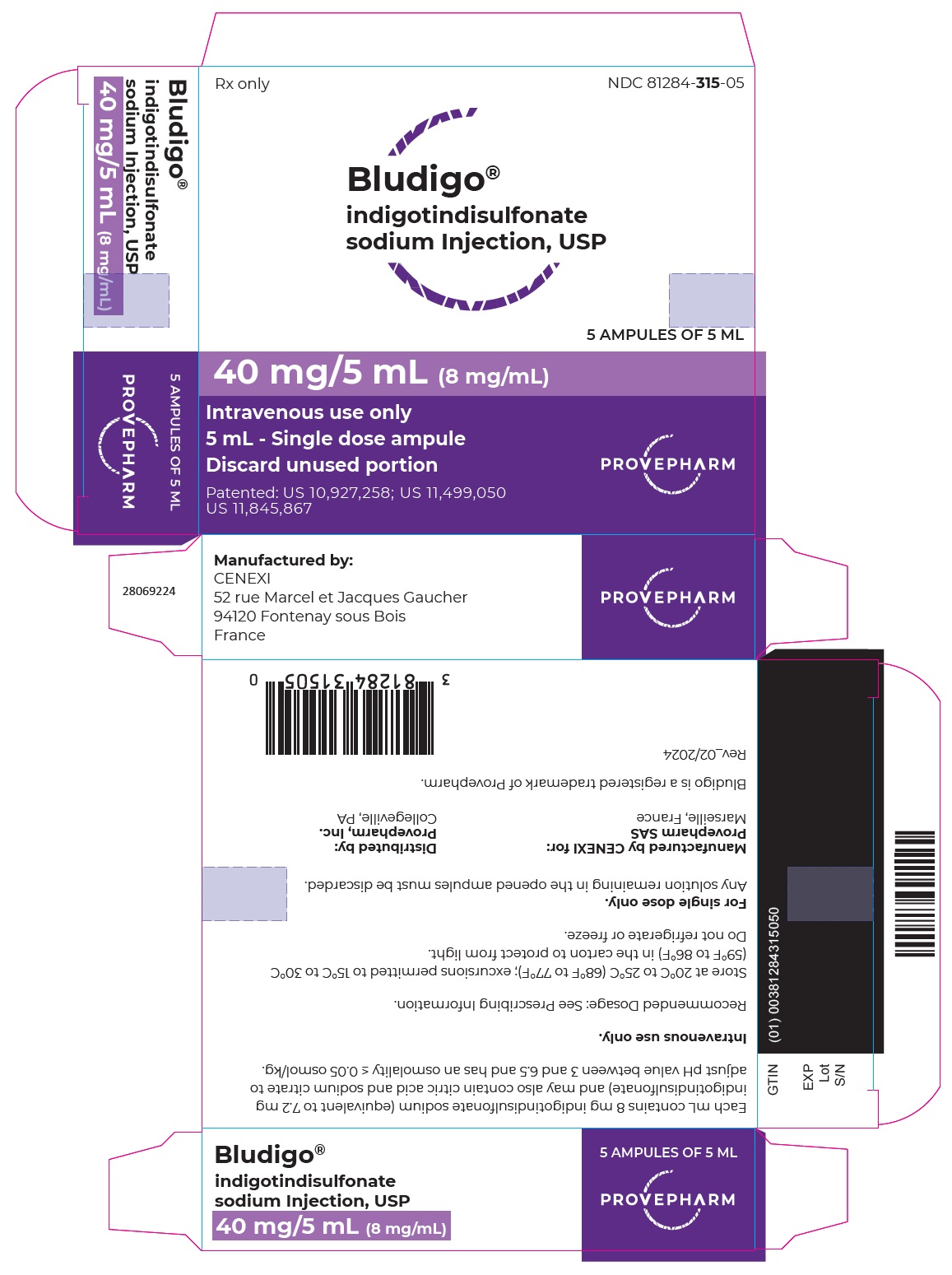
Label: BLUDIGO- indigotindisulfonate sodium injection
- NDC Code(s): 81284-315-00, 81284-315-05
- Packager: Provepharm Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 11, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BLUDIGO safely and effectively. See full prescribing information for BLUDIGO.
BLUDIGO® (indigotindisulfonate sodium injection), for
intravenous use
Initial U.S. Approval: 2022INDICATIONS AND USAGE
BLUDIGO is a diagnostic dye indicated for use as a visualization aid in the cystoscopic assessment of the integrity of the ureters in adults following urological and gynecological open, robotic, or endoscopic surgical procedures. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Injection: 40 mg/5 mL (8 mg/mL) indigotindisulfonate sodium in a single-dose ampule (3)
CONTRAINDICATIONS
- Known hypersensitivity to indigotindisulfonate or any of its components. (4)
WARNINGS AND PRECAUTIONS
- Cardiovascular Reactions: Severe or life-threatening cardiovascular reactions including cardiac arrest, arrhythmia, asystole, atrioventricular block second degree, hypotension, elevation in blood pressure, bradycardia, and tachycardia have been reported. Closely monitor blood pressure and cardiac rhythm during and following the BLUDIGO injection. Interrupt administration if reactions are observed. (5.1)
- Hypersensitivity Reactions: Serious anaphylactic reactions with hypotension, dyspnea, bronchospasm, urticaria, or erythema have been reported. Monitor patients for anaphylactic reactions and have emergency equipment and trained personnel readily available. (5.2)
-
Interference with Oximetry Measurements:
Anesthesiologists should be aware of the potential for artifactual reduction in SpO2 when anesthetized patients are administered BLUDIGO. (5.3)
ADVERSE REACTIONS
Adverse reactions (≥ 1%) are constipation, nausea, vomiting, abdominal pain, pyrexia, ALT increase, and dysuria. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact PROVEPHARM Inc at 1–833-727-6556 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Renal Impairment: BLUDIGO is not recommended for use in patients with eGFR<30 mL/min. (8.6)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Important Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Reactions
5.2 Hypersensitivity Reactions
5.3 Interference with Oximetry Measurements
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dose of BLUDIGO is 5 mL given as an intravenous injection over 1 minute.
The blue color is detectable at the ureteral orifices within 4 minutes to 9 minutes after the intravenous injection.2.2 Important Administration Instructions
- Monitor blood pressure and cardiac rhythm during and following the injection [see Warnings and Precautions (5.1)].
- Use immediately after opening ampule.
- Withdraw the contents of the ampule through a 5 micron or smaller filter straw/filter needle to ensure that the withdrawn solution contains no particulates. The withdrawn solution should be inspected visually for particulate matter and discoloration prior to administration.
- Do not administer with infusion assemblies used with other diluents or drugs.
- Discard any unused portion.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
BLUDIGO is contraindicated in patients with known hypersensitivity to indigotindisulfonate or any of its components [see Warnings and Precautions (5.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Reactions
Severe or life-threatening cardiovascular reactions including cardiac arrest, arrhythmia, asystole, second degree atrioventricular block, hypotension, elevation in blood pressure, bradycardia, and tachycardia have been reported generally within 60 minutes following administration of indigotindisulfonate sodium injection products and required urgent intervention [see Adverse Reactions (6.2)].
Indigotindisulfonate may cause vasoconstriction by interference with vasodilation mediated by nitric oxide dependent mechanisms and by direct vasoconstriction. Indigotindisulfonate may also cause hypotension. Patients with hypertension, heart rate and conduction disorders, or medications causing bradycardia may be at increased risk for elevated blood pressure, hypotension, and bradycardia.
Closely monitor blood pressure and cardiac rhythm during and following the injection of BLUDIGO. Interrupt administration if reactions are observed.
5.2 Hypersensitivity Reactions
Serious anaphylactic reactions with hypotension, dyspnea, bronchospasm, urticaria, or erythema have been reported with the use of indigotindisulfonate sodium injection products [see Adverse Reactions (6.2)]. BLUDIGO is contraindicated in patients with known hypersensitivity to indigotindisulfonate [see Contraindications (4)]. Monitor patients for anaphylactic reactions and have emergency equipment and trained personnel readily available.
5.3 Interference with Oximetry Measurements
Indigotindisulfonate has been reported to interfere with light absorption and transiently interfere with pulse oximetric methods. Anesthesiologists should be aware of the potential for artifactual reduction in SpO2 when anesthetized patients are administered BLUDIGO.
-
6 ADVERSE REACTIONS
Clinically significant adverse reactions are described elsewhere in the labeling:
- Cardiovascular Reactions [see Warnings and Precautions (5.1)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The safety of BLUDIGO was evaluated in a randomized, intra-patient controlled, blind to dose of BLUDIGO, clinical trial. A total of 118 adult patients undergoing endoscopic urological or gynecological procedures were treated intravenously; 58 (49%) of these patients received one dose of BLUDIGO 2.5 mL and 60 (51%) of patients received one dose of BLUDIGO 5 mL. The 2.5 mL dose is not approved [see Dosage and Administration (2.1)]. The mean age of patients was 51 years and 35 (30%) patients were 65 years of age or older. The majority of patients were White 105 (89%) and female 87 (74%).
The adverse reactions (≥1%) reported in the clinical trial are provided in Table 1.
Table 1. Adverse Reactions Reported at ≥1% of Patients Receiving BLUDIGO 5 mL Intravenously
BLUDIGO 5 mL
(N=60)
n (%)Constipation 3 (5.0) Nausea 2 (3.3) Vomiting 2 (3.3) Abdominal Pain 2 (3.3) Pyrexia 2 (3.3) ALT increase 2 (3.3) Dysuria 1 (1.7) 6.2 Postmarketing Experience
The following adverse reactions have been identified following the use of indigotindisulfonate sodium injection products.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular disorders: cardiac arrest, arrhythmia, asystole, atrioventricular block second degree, hypotension, elevation in blood pressure, bradycardia, tachycardia
General disorders and administration site conditions: injection site discolorationImmune system disorders: anaphylactic reactions with hypotension, dyspnea, bronchospasm, urticaria, erythema
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from case reports, case series, observational studies and clinical experience with indigotindisulfonate sodium injection use in pregnant women over several decades have not identified a drug associated risk of adverse maternal and fetal adverse effects. Indigotindisulfonate sodium injection use during the first trimester of pregnancy is rare; thus, the data are insufficient to evaluate for a drug associated risk of major birth defects and miscarriage. The majority of the published data were from intra-amniotic injections. Animal reproduction studies using the intravenous route of administration have not been conducted. Oral administration of indigotindisulfonate sodium to pregnant rats and rabbits produced no evidence of fetal harm.
However, oral availability is low (3%) so that the risk of intravenous administration of indigotindisulfonate sodium during pregnancy cannot be evaluated from the data available .
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.8.2 Lactation
Risk Summary
It is not known if indigotindisulfonate is present in animal or human milk. The transfer of indigotindisulfonate into breastmilk is likely to be low and adverse effects on the breastfed infant are not expected [see Clinical Pharmacology (12.3)]. There are no data on the effect of indigotindisulfonate on the breastfed infant or milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for BLUDIGO and any potential adverse effects on the breastfed infant from BLUDIGO or from the underlying maternal condition.8.4 Pediatric Use
The safety and effectiveness of BLUDIGO have not been established in pediatric patients.
8.5 Geriatric Use
Of the total number of subjects in the clinical study of BLUDIGO, 23 (20%) were 65 to 74 years of age, and 12 (10%) were 75 and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
Indigotindisulfonate is known to be excreted by the kidney through tubular secretion. No dedicated pharmacokinetic study using BLUDIGO in patients with varying degree of renal impairment has been conducted. Based on subgroup analyses in the randomized clinical trial, dose adjustment is not needed in patients with mild to moderate renal impairment (estimated glomerular filtration rate (eGFR) 30 to 89 mL/min derived from the Modification of Diet in Renal Disease formula).
BLUDIGO has not been studied in patients with eGFR < 30 mL/min and is not recommended for use in these patients.
-
11 DESCRIPTION
BLUDIGO (indigotindisulfonate sodium injection, USP) is a sterile deep blue or bluish-purple diagnostic dye for intravenous use.
Each mL contains 8 mg indigotindisulfonate sodium (equivalent to 7.2 mg indigotindisulfonate) and may also contain citric acid and sodium citrate to adjust pH value between 3 and 6.5 and has an osmolality ≤ 0.05 osmol/kg. Indigotindisulfonate sodium is also known as indigo carmine.
The chemical name of indigotindisulfonate sodium is Disodium 3,3’-dioxo-[Δ2,2’-biindoline]-5,5’-disulfonate. Its molecular formula is C16H8N2Na2O8S2 and its molecular weight is 466.35 g/mol. Its structural formula is: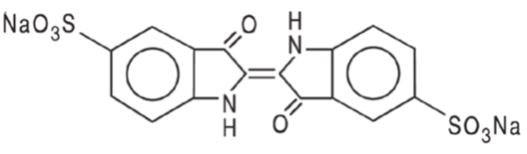
indigotindisulfonate sodium
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Indigotindisulfonate is a dye excreted by the kidney through tubular secretion and enhances visualization of the ureteral orifices by its deep blue color.
12.2 Pharmacodynamics
The blue color is detectable at the ureteral orifices within 4 minutes to 9 minutes after the intravenous injection.
12.3 Pharmacokinetics
The pharmacokinetic properties of BLUDIGO are presented in Table 2.
Table 2. Pharmacokinetics of Indigotindisulfonate in Healthy Adults Following Intravenous Administration of
BLUDIGO 5 mL (40 mg)BLUDIGO
5 mL (40 mg)
General Information
Cmax (CV%), μg/mL
6.33 (58.4)
AUC0-INF (CV%), μg·h/mL
1.15 (36.4)
Distribution
Mean (CV%) Volume of Distribution, L
10.7 (36.1)
Protein Binding, in vitro
About 90%
Elimination
Mean (CV%) Elimination Half-life, minutes
12 (34.1)
Mean (CV%) Clearance, L/hour
Urinary
7.08 (66.3)
Total
40.2 (45.1)
Metabolism
Primary Metabolic Pathways
Oxidative metabolism
Excretion
Urine (CV%)a
16.0% (44.0)
Feces
<2%
aUnchanged drug
Cmax= maximum plasma concentration; AUC0-INF=area under the plasma concentration-time curve from time of administration extrapolated to infinity; CV=coefficient of variation
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
Carcinogenicity studies in animals have not been conducted with indigotindisulfonate sodium using the intravenous route of administration.
Long-term studies in mice with oral and subcutaneous administration of indigotindisulfonate sodium revealed no carcinogenic effects.Mutagenesis
Although indigotindisulfonate sodium has been evaluated in a number of Ames assay studies, an Ames assay study that follows all currently recommended guidelines has not been performed. Indigotindisulfonate was not genotoxic in all those Ames assays.
The mutagenicity of indigotindisulfonate was inconclusive in the in vitro mouse L5187Y Lymphoma TK +/- assay. Orally administered indigotindisulfonate was not mutagenic in the in vivo mouse micronucleus test. An in vivo micronucleus test with indigotindisulfonate sodium using the intravenous route of administration has not been conducted.Fertility
Fertility studies with indigotindisulfonate sodium using the intravenous route of administration have not been conducted. -
14 CLINICAL STUDIES
The safety and efficacy of BLUDIGO were evaluated in a randomized intra-patient controlled, blind to dose of BLUDIGO, multi-center study (NCT04228445) in 118 adult patients undergoing endoscopic urological or gynecological surgical procedures including cystoscopic (58%), robotic (28%), and transvaginal (14%) approaches.
The majority of patients were white (89%), female (74%), and younger than 65 years of age (70%). Mean age was 51 years, and age ranged from 20 to 88 years.
Patients were randomized in a 1:1 ratio to receive 2.5 mL or 5 mL of BLUDIGO intravenously prior to the end of the surgical procedure. Each patient underwent cystoscopy and received 5 mL of sodium chloride injection 0.9% followed by the randomized BLUDIGO dose for visualization of urinary flow from the ureteral orifices. The 2.5 mL dose is not approved [see Dosage and Administration (2.1)].
The ureteral orifices and urine flow were observed and video recorded from 0 to 10 minutes post injection, with separate recordings made following sodium chloride injection and BLUDIGO. The conspicuity of the urine flow from the ureteral orifices was assessed in a randomized, blinded fashion by two independent central reviewers using a 5-point scale (1 = no urine flow observed; 2 = weak urine flow, little color contrast; 3 = Color contrast or significant urine flow; 4 = strong urine flow with good color contrast; and 5 = strong urine flow with striking contrast in color) once after sodium chloride injection and twice after BLUDIGO resulting in paired data. The surgeons also reviewed and scored conspicuity of the urine flow from the ureteral orifices. The responder for a ureter is defined as the difference in conspicuity score between BLUDIGO and sodium chloride injection being at least one point difference and the conspicuity score following BLUDIGO alone being greater than or equal to three. The reviewer agreement with the responder endpoint is acceptable. The proportion of responders along with its 95% confidence interval by ureter and reviewer or surgeon is summarized in Table 3.
Table 3. Summary of Proportion of Responders by Ureter and Reviewer or Surgeon in Patients Receiving BLUDIGO 5 mLBLUDIGO 5 mL
Left Ureter (n=49)
Right Ureter (n=49)
Reviewer 1
% Responder*
95% CL**
63%
(48%, 77%)
76%
(61%, 87%)
Reviewer 2
% responder
95% CL
78%
(63%, 88%)
82%
(68%, 91%)
Surgeon
% responder
95% CL
71%
(57%, 83%)
82%
(68%, 91%)
*responder: IC conspicuity score ≥ 3 and difference (IC – Saline) in conspicuity score ≥1, missing data is imputed as non-responder
** two-sided 95% confidence limits for the proportion of responder, calculated using the Clopper-Pearson (Exact) method
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
BLUDIGO (indigotindisulfonate sodium injection, USP) 40 mg/5 mL (8 mg/mL) is a dark blue or bluish-purple solution supplied in a carton of 5 single-dose amber glass ampules (NDC 81284-315-05).
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
Store in original carton to protect from light.
Do not refrigerate or freeze.
Use immediately after opening ampule. Discard unused portion. -
17 PATIENT COUNSELING INFORMATION
Cardiovascular Reactions
Advise the patient of the possibility of developing elevated blood pressure, hypotension, bradycardia, tachycardia, or atrioventricular block during or after the administration of BLUDIGO [see Warnings and Precautions (5.1)].Injection Site and Urine Discoloration
Inform the patient that BLUDIGO may cause a blue discoloration of injection site and urine and that the discoloration should resolve within 48 hours. Advise the patient to inform the healthcare provider if the discoloration of the injection site is associated with other symptoms [see Adverse Reactions (6.2)]. - PRINCIPAL DISPLAY PANEL - CARTON
- PRINCIPAL DISPLAY PANEL - VIAL
-
INGREDIENTS AND APPEARANCE
BLUDIGO
indigotindisulfonate sodium injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:81284-315 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INDIGOTINDISULFONATE SODIUM (UNII: D3741U8K7L) (INDIGOTINDISULFONIC ACID - UNII:X7OI7JF73P) INDIGOTINDISULFONATE SODIUM 8 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81284-315-05 5 in 1 CARTON 09/06/2022 1 NDC:81284-315-00 5 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216264 09/06/2022 Labeler - Provepharm Inc. (086861066) Establishment Name Address ID/FEI Business Operations CENEXI 573309239 manufacture(81284-315) , pack(81284-315) , analysis(81284-315)