Label: YMLABS- spf30 flavored lip balm stick
-
Contains inactivated NDC Code(s)
NDC Code(s): 10827-0008-1 - Packager: Yusef Manufacturing Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 25, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
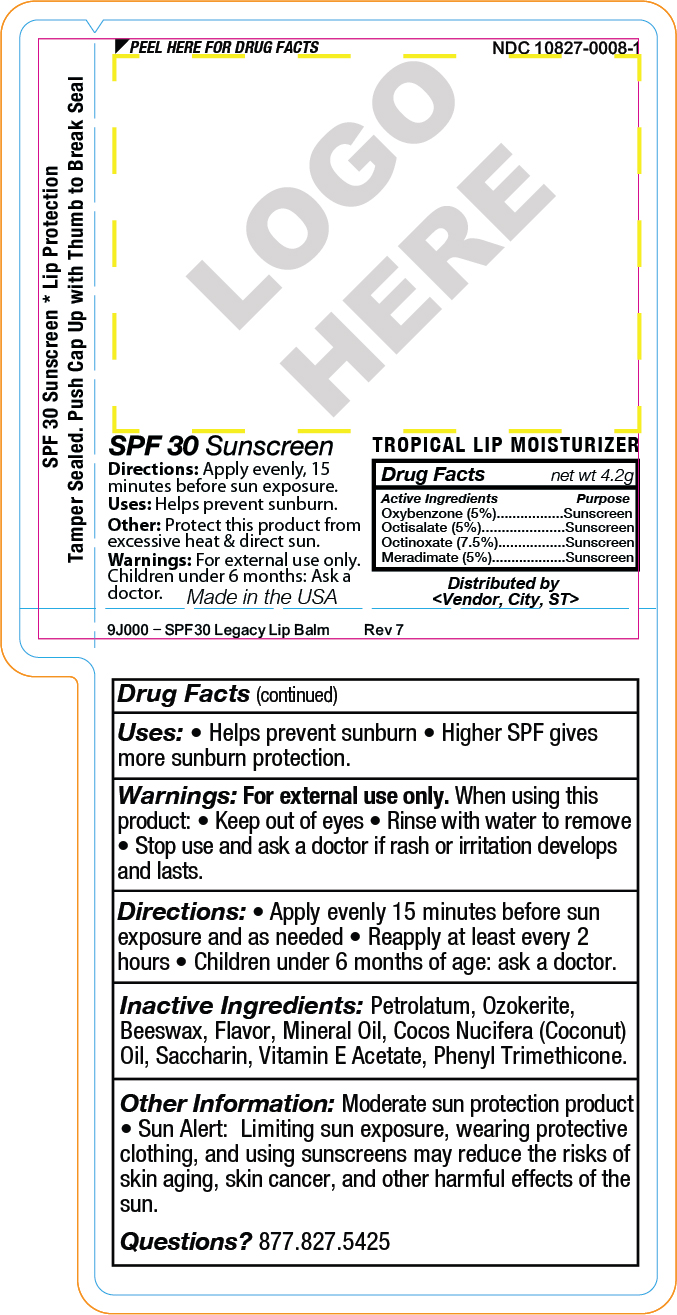
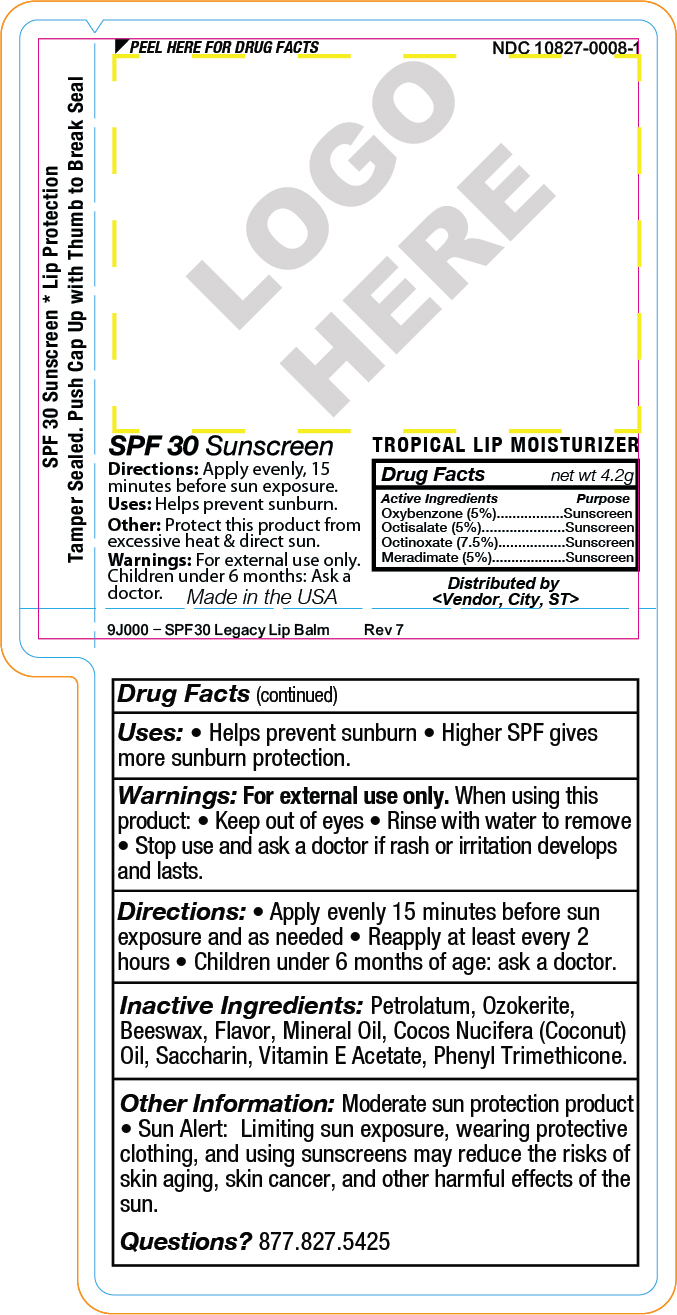
SPF 30 Sunscreen
Uses: Helps prevent sunburn.
Directions: Apply evenly, 15
minutes before sun exposure.
Other: Protect this product from
excessive heat & direct sun.
Warnings: For external use only.
Children under 6 months: Ask a
doctor.Drug Facts net wt 4.2g
Active Ingredients PurposeMeradimate (5%)...........Sunscreen
Octinoxate (7.5%).............Sunscreen
Octisalate (5%)..............Sunscreen
Oxybenzone (5%)...........SunscreenMade in the USA
Distributed by
<Vendor, City, ST> - SPL UNCLASSIFIED SECTION
- WARNINGS
- INSTRUCTIONS FOR USE
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
YMLABS
spf30 flavored lip balm stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10827-0008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MERADIMATE (UNII: J9QGD60OUZ) (MERADIMATE - UNII:J9QGD60OUZ) MERADIMATE 0.21 g in 4.2 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.315 g in 4.2 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 0.21 g in 4.2 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 0.21 g in 4.2 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) CERESIN (UNII: Q1LS2UJO3A) WHITE WAX (UNII: 7G1J5DA97F) MINERAL OIL (UNII: T5L8T28FGP) COCONUT OIL (UNII: Q9L0O73W7L) SACCHARIN (UNII: FST467XS7D) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10827-0008-1 4.2 g in 1 TUBE; Type 0: Not a Combination Product 12/30/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 12/30/2021 Labeler - Yusef Manufacturing Laboratories (144150674) Registrant - Yusef Manufacturing Laboratories (144150674) Establishment Name Address ID/FEI Business Operations Yusef Manufacturing Laboratories 144150674 manufacture(10827-0008)