Label: AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 8B PORCELAIN BEIGE- titanium dioxide and zinc oxide liquid
AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 22G LIGHT GOLDEN- titanium dioxide and zinc oxide liquid
AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 37S MEDIUM-TAN SAND .......CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 51S DEEP SAND- titanium dioxide and zinc oxide liquid
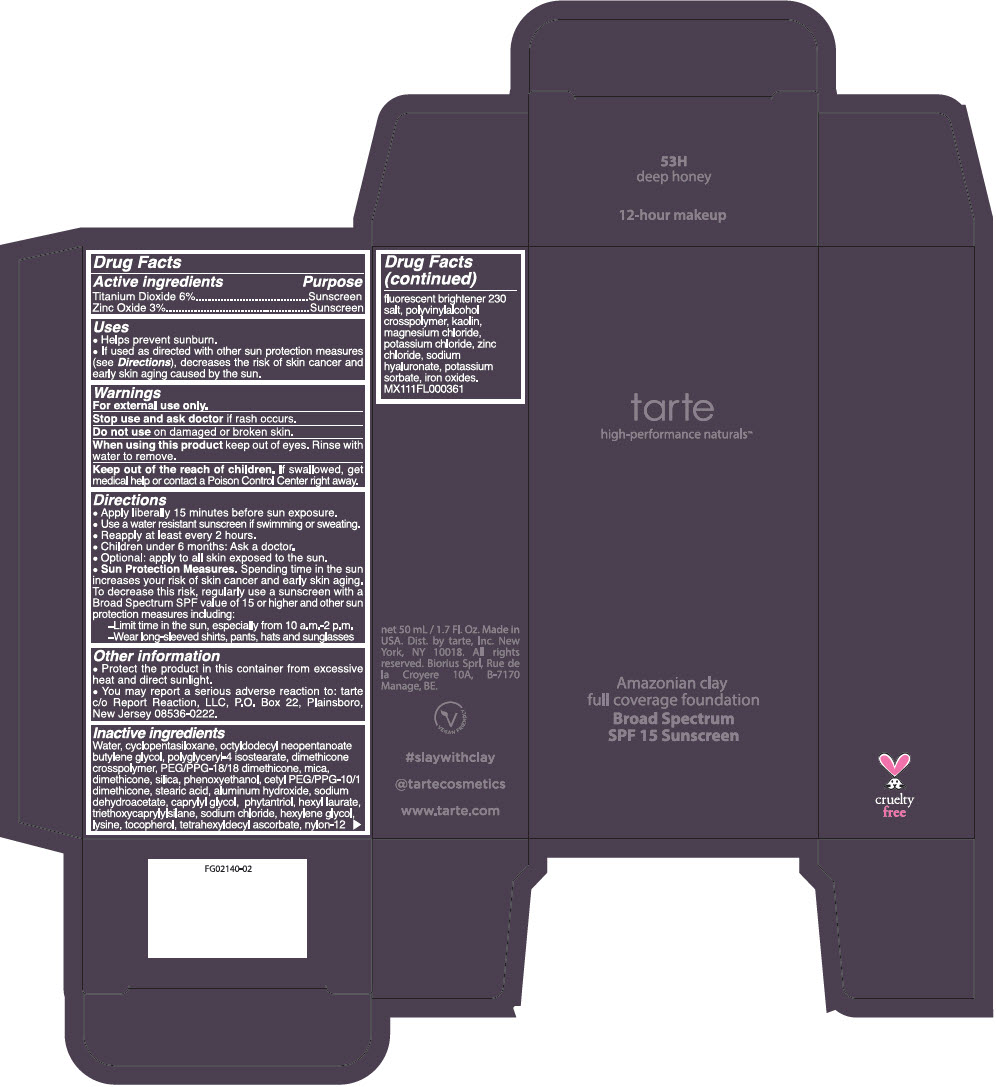
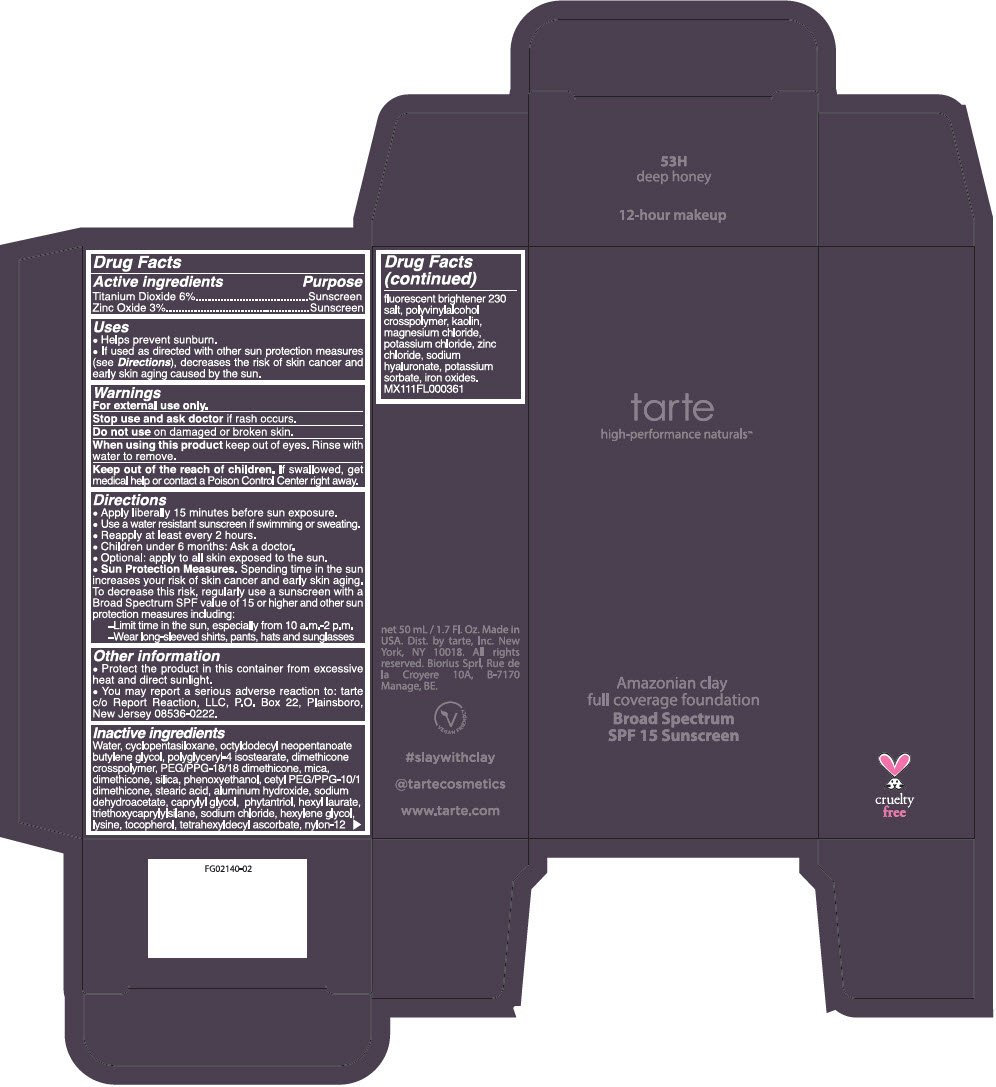
AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 53H DEEP HONEY- titanium dioxide and zinc oxide liquid
AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 57H RICH HONEY- titanium dioxide and zinc oxide liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 51060-127-01, 51060-128-01, 51060-129-01, 51060-130-01, view more51060-131-01, 51060-132-01, 51060-133-01, 51060-134-01, 51060-135-01, 51060-136-01, 51060-137-01, 51060-138-01, 51060-139-01, 51060-140-01, 51060-141-01, 51060-142-01, 51060-143-01, 51060-144-01, 51060-145-01, 51060-146-01, 51060-147-01, 51060-148-01, 51060-149-01, 51060-150-01, 51060-151-01, 51060-152-01, 51060-153-01, 51060-154-01, 51060-155-01, 51060-156-01, 51060-157-01, 51060-158-01, 51060-159-01, 51060-160-01, 51060-161-01, 51060-162-01, 51060-163-01, 51060-164-01, 51060-165-01, 51060-166-01 - Packager: Tarte, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 17, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure.
- Use a water resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
- Children under 6 months: Ask a doctor.
- Optional: apply to all skin exposed to the sun.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- –
- Limit time in the sun, especially from 10 a.m.-2 p.m.
- –
- Wear long-sleeved shirts, pants, hats and sunglasses
- Other information
-
Inactive ingredients
Water, cyclopentasiloxane, octyldodecyl neopentanoate butylene glycol, polyglyceryl-4 isostearate, dimethicone crosspolymer, PEG/PPG-18/18 dimethicone, mica, dimethicone, silica, phenoxyethanol, cetyl PEG/PPG-10/1 dimethicone, stearic acid, aluminum hydroxide, sodium dehydroacetate, caprylyl glycol, phytantriol, hexyl laurate, triethoxycaprylylsilane, sodium chloride, hexylene glycol, lysine, tocopherol, tetrahexyldecyl ascorbate, nylon-12 fluorescent brightener 230 salt, polyvinylalcohol crosspolymer, kaolin, magnesium chloride, potassium chloride, zinc chloride, sodium hyaluronate, potassium sorbate, iron oxides. MX111FL000361.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 8B Porcelain Beige
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 22G Light Golden
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 37S Medium-Tan Sand
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 37B Medium-Tan Beige
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 39N Medium-Tan Neutral
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 42G Tan Golden
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 42N Tan Neutral
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 47G Tan-Deep Golden
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 48N Tan-Deep Neutral
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 51G Deep Golden
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 51N Deep Neutral
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 56G Rich Golden
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 58N Rich Neutral
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 57S Rich Sand
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 60H Mahogany
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 12S Fair Sand
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 12B Fair Beige
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 13N Ivory
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 16N Fair-light Neutral
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 16B Fair-light Beige
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 16H Fair-light Honey
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 22S Light Sand
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 22N Light Neutral
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 22B Light Beige
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 27S Light-Medium Sand
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 27N Light-medium Neutral
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 29B Light-medium Beige
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 29H Light-medium Honey
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 35S Medium Sand
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 35N Medium Neutral
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 35B Medium Beige
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 35H Medium Honey
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 36H Medium-tan Honey
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 40H Tan Honey
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 44S Tan Sand
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 46S Tan-deep Sand
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 47H Tan-deep Honey
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 51S Deep Sand
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 53H Deep Honey
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton - 57H Rich Honey
-
INGREDIENTS AND APPEARANCE
AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 8B PORCELAIN BEIGE
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-127 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) MICA (UNII: V8A1AW0880) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE (UNII: 92RU3N3Y1O) HEXYL LAURATE (UNII: 4CG9F9W01Q) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) PHYTANTRIOL (UNII: 8LVI07A72W) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) NYLON-12 (UNII: 446U8J075B) SODIUM CHLORIDE (UNII: 451W47IQ8X) OCTYLDODECANOL (UNII: 461N1O614Y) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) METHICONE (20 CST) (UNII: 6777U11MKT) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-127-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 22G LIGHT GOLDEN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-128 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) MICA (UNII: V8A1AW0880) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE (UNII: 92RU3N3Y1O) HEXYL LAURATE (UNII: 4CG9F9W01Q) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) PHYTANTRIOL (UNII: 8LVI07A72W) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) NYLON-12 (UNII: 446U8J075B) SODIUM CHLORIDE (UNII: 451W47IQ8X) OCTYLDODECANOL (UNII: 461N1O614Y) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) METHICONE (20 CST) (UNII: 6777U11MKT) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-128-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 37S MEDIUM-TAN SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-129 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) MICA (UNII: V8A1AW0880) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE (UNII: 92RU3N3Y1O) HEXYL LAURATE (UNII: 4CG9F9W01Q) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) PHYTANTRIOL (UNII: 8LVI07A72W) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) NYLON-12 (UNII: 446U8J075B) SODIUM CHLORIDE (UNII: 451W47IQ8X) OCTYLDODECANOL (UNII: 461N1O614Y) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) METHICONE (20 CST) (UNII: 6777U11MKT) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-129-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 37B MEDIUM-TAN BEIGE
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-130 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) MICA (UNII: V8A1AW0880) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE (UNII: 92RU3N3Y1O) HEXYL LAURATE (UNII: 4CG9F9W01Q) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) PHYTANTRIOL (UNII: 8LVI07A72W) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) NYLON-12 (UNII: 446U8J075B) SODIUM CHLORIDE (UNII: 451W47IQ8X) OCTYLDODECANOL (UNII: 461N1O614Y) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) METHICONE (20 CST) (UNII: 6777U11MKT) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-130-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 39N MEDIUM-TAN NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-131 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) MICA (UNII: V8A1AW0880) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE (UNII: 92RU3N3Y1O) HEXYL LAURATE (UNII: 4CG9F9W01Q) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) PHYTANTRIOL (UNII: 8LVI07A72W) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) NYLON-12 (UNII: 446U8J075B) SODIUM CHLORIDE (UNII: 451W47IQ8X) OCTYLDODECANOL (UNII: 461N1O614Y) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) METHICONE (20 CST) (UNII: 6777U11MKT) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-131-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 42G TAN GOLDEN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-132 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) MICA (UNII: V8A1AW0880) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE (UNII: 92RU3N3Y1O) HEXYL LAURATE (UNII: 4CG9F9W01Q) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) PHYTANTRIOL (UNII: 8LVI07A72W) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) NYLON-12 (UNII: 446U8J075B) SODIUM CHLORIDE (UNII: 451W47IQ8X) OCTYLDODECANOL (UNII: 461N1O614Y) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) METHICONE (20 CST) (UNII: 6777U11MKT) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-132-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 42N TAN NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-133 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) MICA (UNII: V8A1AW0880) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE (UNII: 92RU3N3Y1O) HEXYL LAURATE (UNII: 4CG9F9W01Q) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) PHYTANTRIOL (UNII: 8LVI07A72W) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) NYLON-12 (UNII: 446U8J075B) SODIUM CHLORIDE (UNII: 451W47IQ8X) OCTYLDODECANOL (UNII: 461N1O614Y) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) METHICONE (20 CST) (UNII: 6777U11MKT) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-133-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 47G TAN-DEEP GOLDEN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-134 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) MICA (UNII: V8A1AW0880) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE (UNII: 92RU3N3Y1O) HEXYL LAURATE (UNII: 4CG9F9W01Q) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) PHYTANTRIOL (UNII: 8LVI07A72W) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) NYLON-12 (UNII: 446U8J075B) SODIUM CHLORIDE (UNII: 451W47IQ8X) OCTYLDODECANOL (UNII: 461N1O614Y) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) METHICONE (20 CST) (UNII: 6777U11MKT) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-134-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 48N TAN-DEEP NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-135 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) MICA (UNII: V8A1AW0880) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE (UNII: 92RU3N3Y1O) HEXYL LAURATE (UNII: 4CG9F9W01Q) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) PHYTANTRIOL (UNII: 8LVI07A72W) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) NYLON-12 (UNII: 446U8J075B) SODIUM CHLORIDE (UNII: 451W47IQ8X) OCTYLDODECANOL (UNII: 461N1O614Y) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) METHICONE (20 CST) (UNII: 6777U11MKT) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-135-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 51G DEEP GOLDEN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-136 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) MICA (UNII: V8A1AW0880) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE (UNII: 92RU3N3Y1O) HEXYL LAURATE (UNII: 4CG9F9W01Q) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) PHYTANTRIOL (UNII: 8LVI07A72W) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) NYLON-12 (UNII: 446U8J075B) SODIUM CHLORIDE (UNII: 451W47IQ8X) OCTYLDODECANOL (UNII: 461N1O614Y) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) METHICONE (20 CST) (UNII: 6777U11MKT) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-136-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 51N DEEP NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-137 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) MICA (UNII: V8A1AW0880) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE (UNII: 92RU3N3Y1O) HEXYL LAURATE (UNII: 4CG9F9W01Q) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) PHYTANTRIOL (UNII: 8LVI07A72W) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) NYLON-12 (UNII: 446U8J075B) SODIUM CHLORIDE (UNII: 451W47IQ8X) OCTYLDODECANOL (UNII: 461N1O614Y) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) METHICONE (20 CST) (UNII: 6777U11MKT) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-137-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 56G RICH GOLDEN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-138 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) MICA (UNII: V8A1AW0880) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE (UNII: 92RU3N3Y1O) HEXYL LAURATE (UNII: 4CG9F9W01Q) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) PHYTANTRIOL (UNII: 8LVI07A72W) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) NYLON-12 (UNII: 446U8J075B) SODIUM CHLORIDE (UNII: 451W47IQ8X) OCTYLDODECANOL (UNII: 461N1O614Y) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) METHICONE (20 CST) (UNII: 6777U11MKT) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-138-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 58N RICH NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-139 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) MICA (UNII: V8A1AW0880) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE (UNII: 92RU3N3Y1O) HEXYL LAURATE (UNII: 4CG9F9W01Q) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) PHYTANTRIOL (UNII: 8LVI07A72W) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) NYLON-12 (UNII: 446U8J075B) SODIUM CHLORIDE (UNII: 451W47IQ8X) OCTYLDODECANOL (UNII: 461N1O614Y) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) METHICONE (20 CST) (UNII: 6777U11MKT) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-139-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 57S RICH SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-140 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) MICA (UNII: V8A1AW0880) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE (UNII: 92RU3N3Y1O) HEXYL LAURATE (UNII: 4CG9F9W01Q) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) PHYTANTRIOL (UNII: 8LVI07A72W) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) NYLON-12 (UNII: 446U8J075B) SODIUM CHLORIDE (UNII: 451W47IQ8X) OCTYLDODECANOL (UNII: 461N1O614Y) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) METHICONE (20 CST) (UNII: 6777U11MKT) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-140-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 60H MAHOGANY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-141 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) MICA (UNII: V8A1AW0880) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE (UNII: 92RU3N3Y1O) HEXYL LAURATE (UNII: 4CG9F9W01Q) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) PHYTANTRIOL (UNII: 8LVI07A72W) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) NYLON-12 (UNII: 446U8J075B) SODIUM CHLORIDE (UNII: 451W47IQ8X) OCTYLDODECANOL (UNII: 461N1O614Y) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) METHICONE (20 CST) (UNII: 6777U11MKT) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-141-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 12S FAIR SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-142 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-142-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 12B FAIR BEIGE
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-143 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-143-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 13N IVORY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-144 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-144-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 16N FAIR-LIGHT NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-145 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-145-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 16B FAIR-LIGHT BEIGE
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-146 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-146-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 16H FAIR-LIGHT HONEY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-147 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-147-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 22S LIGHT SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-148 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-148-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 22N LIGHT NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-149 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-149-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 22B LIGHT BEIGE
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-150 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-150-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 27S LIGHT-MEDIUM SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-151 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-151-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 27N LIGHT-MEDIUM NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-152 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-152-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 29B LIGHT-MEDIUM BEIGE
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-153 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-153-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 29H LIGHT-MEDIUM HONEY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-154 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-154-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 35S MEDIUM SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-155 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-155-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 35N MEDIUM NEUTRAL
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-156 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-156-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 35B MEDIUM BEIGE
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-157 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-157-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 35H MEDIUM HONEY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-158 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-158-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 36H MEDIUM-TAN HONEY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-159 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-159-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 40H TAN HONEY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-160 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-160-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 44S TAN SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-161 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-161-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 46S TAN-DEEP SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-162 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-162-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 47H TAN-DEEP HONEY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-163 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-163-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 51S DEEP SAND
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-164 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-164-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 53H DEEP HONEY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-165 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-165-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 AMAZONIAN CLAY 12-HOUR FULL COVERAGE FOUNDATION BROAD SPECTRUM SPF 15 SUNSCREEN 57H RICH HONEY
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-166 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 60 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) MICA (UNII: V8A1AW0880) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYL LAURATE (UNII: 4CG9F9W01Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CHLORIDE (UNII: 451W47IQ8X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) LYSINE (UNII: K3Z4F929H6) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-166-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2018 Labeler - Tarte, Inc (027905186)