Label: HYDROXYZINE HYDROCHLORIDE injection, solution
- NDC Code(s): 0404-9877-01
- Packager: Henry Schein, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0517-5601
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Description
Hydroxyzine hydrochloride has the chemical name of (±)-2-[2-[4-(p-Chloro-a phenylbenzyl) -1-piperazinyl]ethoxy]ethanol dihydrochloride and occurs as a white, odorless powder which is very soluble in water.
It has the following structural formula:
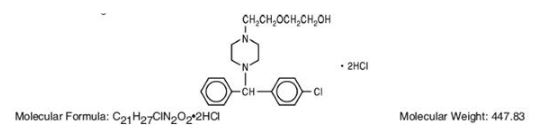
Molecular Formula: C21H27CIN202•2HCI Molecular Weight: 447.83
Hydroxyzine Hydrochloride Injection, USP is a sterile aqueous solution intended for intramuscular administration.
Each mL contains: Hydroxyzine HCI 25 mg or 50 mg, Benzyl Alcohol 0.9%, and Water for Injection q.s. pH adjusted with Sodium Hydroxide and/or Hydrochloric Acid.
-
Clinical Pharmacology
Hydroxyzine hydrochloride is unrelated chemically to phenothiazine, reserpine, and meprobamate. Hydroxyzine has demonstrated its clinical effectiveness in the chemotherapeutic aspect of the total management of neuroses and emotional disturbances manifested by anxiety, tension, agitation, apprehension or confusion.
Hydroxyzine has been shown clinically to be a rapid-acting true ataraxic with a wide margin of safety. It induces a calming effect in anxious, tense, psychoneurotic adults and also in anxious, hyperkinetic children without impairing mental alertness. It is not a cortical depressant, but its action may be due to a suppression of activity in certain key regions of the subcortical area of the central nervous system.
Primary skeletal muscle relaxation has been demonstrated experimentally.
Hydroxyzine has been shown experimentally to have antispasmodic properties, apparently mediated through interference with the mechanism that responds to spasmogenic agents such as serotonin, acetylcholine, and histamine.
Antihistaminic effects have been demonstrated experimentally and confirmed clinically.
An antiemetic effect, both by the apomorphine test and the veriloid test, has been demonstrated. Pharmacological and clinical studies indicate that hydroxyzine in therapeutic dosage does not increase gastric secretion or acidity and in most cases provides mild antisecretory benefits.
-
Indications and Usage
The total management of anxiety, tension, and psychomotor agitation in conditions of emotional stress requires in most instances a combined approach of psychotherapy and chemotherapy. Hydroxyzine has been found to be particularly useful for this latter phase of therapy in its ability to render the disturbed patient more amenable to psychotherapy in long term treatment of the psychoneurotic and psychotic, although it should not be used as the sole treatment of psychosis or of clearly demonstrated cases of depression.
Hydroxyzine is also useful in alleviating the manifestations of anxiety and tension as in the preparation for dental procedures and in acute emotional problems. It has also been recommended for the management of anxiety associated with organic disturbances and as adjunctive therapy in alcoholism and allergic conditions with strong emotional overlay, such as in asthma, chronic urticaria, and pruritus.
Hydroxyzine hydrochloride intramuscular solution is useful in treating the following types of patients when intramuscular administration is indicated:
1. The acutely disturbed or hysterical patient.
2. The acute or chronic alcoholic with anxiety withdrawal symptoms or delirium tremens.
3. As pre- and postoperative and pre- and postpartum adjunctive medication to permit reduction in narcotic dosage, allay anxiety and control emesis.
Hydroxyzine hydrochloride has also demonstrated effectiveness in controlling nausea and vomiting, excluding nausea and vomiting of pregnancy. (See CONTRAINDICATIONS).
In prepartum states, the reduction in narcotic requirement effected by hydroxyzine is of particular benefit to both mother and neonate.
Hydroxyzine benefits the cardiac patient by its ability to allay the associated anxiety and apprehension attendant to certain types of heart disease. Hydroxyzine is not known to interfere with the action of digitalis in any way and may be used concurrently with this agent.
The effectiveness of hydroxyzine in long term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should reassess periodically the usefulness of the drug for the individual patient.
-
Contraindications
Hydroxyzine hydrochloride intramuscular solution is intended only for intramuscular administration and should not, under any circumstances, be injected subcutaneously, intra arterially or intravenously.
Hydroxyzine is contraindicated in patients with a prolonged QT interval.
This drug is contraindicated for patients who have shown a previous hypersensitivity to it.
Hydroxyzine, when administered to the pregnant mouse, rat, and rabbit, induced fetal abnormalities in the rat at doses substantially above the human therapeutic range. Clinical data in human beings are inadequate to establish safety in early pregnancy. Until such data are available, hydroxyzine is contraindicated in early pregnancy.
- Warnings
-
Adverse Reactions
Therapeutic doses of hydroxyzine seldom produce impairment of mental alertness. However, drowsiness may occur; if so, it is usually transitory and may disappear in a few days of continued therapy or upon reduction of the dose. Dryness of the mouth may be encountered at higher doses. Extensive clinical use has substantiated the absence of toxic effects on the liver or bone marrow when administered in the recommended doses for over four years of uninterrupted therapy. The absence of adverse effects has been further demonstrated in experimental studies in which excessively high doses were administered.
Involuntary motor activity, including rare instances of tremor and convulsions, has been reported, usually with doses considerably higher than those recommended. Continuous therapy with over one gram per day has been employed in some patients without these effects having been encountered. Hydroxyzine hydrochloride is associated with Acute Generalized Exanthematous Pustulosis (AGEP) in post marketing reports.
- SPL UNCLASSIFIED SECTION
-
Dosage and Administration
The recommended dosages for hydroxyzine hydrochloride intramuscular solution are:
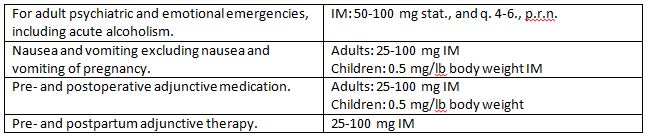
As with all potent medications, the dosage should be adjusted according to the patient's response to therapy.
FOR ADDITIONAL INFORMATION ON THE ADMINISTRATION AND SITE OF SELECTION SEE PRECAUTIONS SECTION. NOTE: Hydroxyzine hydrochloride intramuscular solution may be administered without further dilution.
Patients may be started on intramuscular therapy when indicated. They should be maintained on oral therapy whenever this route is practicable.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
-
How Supplied
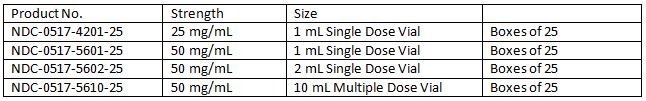
Storage Condition: Store at 20° to 25°C (68° to 7JOF); excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled Room Temperature). Protect from light. Discard unused portion of the single dose vial.
Product repackaged by: Henry Schein, Inc., Bastian, VA 24314 From Original Manufacturer/Distributor's NDC and Unit of Sale To Henry Schein Repackaged Product NDC and Unit of Sale Total Strength/Total Volume (Concentration) per unit NDC 0517-5601-25
Boxes of 25NDC 0404-9877-01
1 1mL Single Dose Vial in a bag
(Vial bears NDC 0517-5601-25)50 mg/mL NDC 0517-5602-25
Boxes of 25NDC 0404-9878-02
1 2 mL Single Dose Vial in a bag
(Vial bears NDC 0517-5602-25)50 mg/mL AMERICAN REGENT, INC.
SHIRLEY, NY 11967
IN4201
Rev. 10/16
MG #7842
- SAMPLE PACKAGE LABEL
-
INGREDIENTS AND APPEARANCE
HYDROXYZINE HYDROCHLORIDE
hydroxyzine hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0404-9877(NDC:0517-5601) Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROXYZINE DIHYDROCHLORIDE (UNII: 76755771U3) (Hydroxyzine - UNII:30S50YM8OG) HYDROXYZINE DIHYDROCHLORIDE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0404-9877-01 1 in 1 BAG 01/17/2022 1 1 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA087408 01/17/2022 Labeler - Henry Schein, Inc. (012430880)


