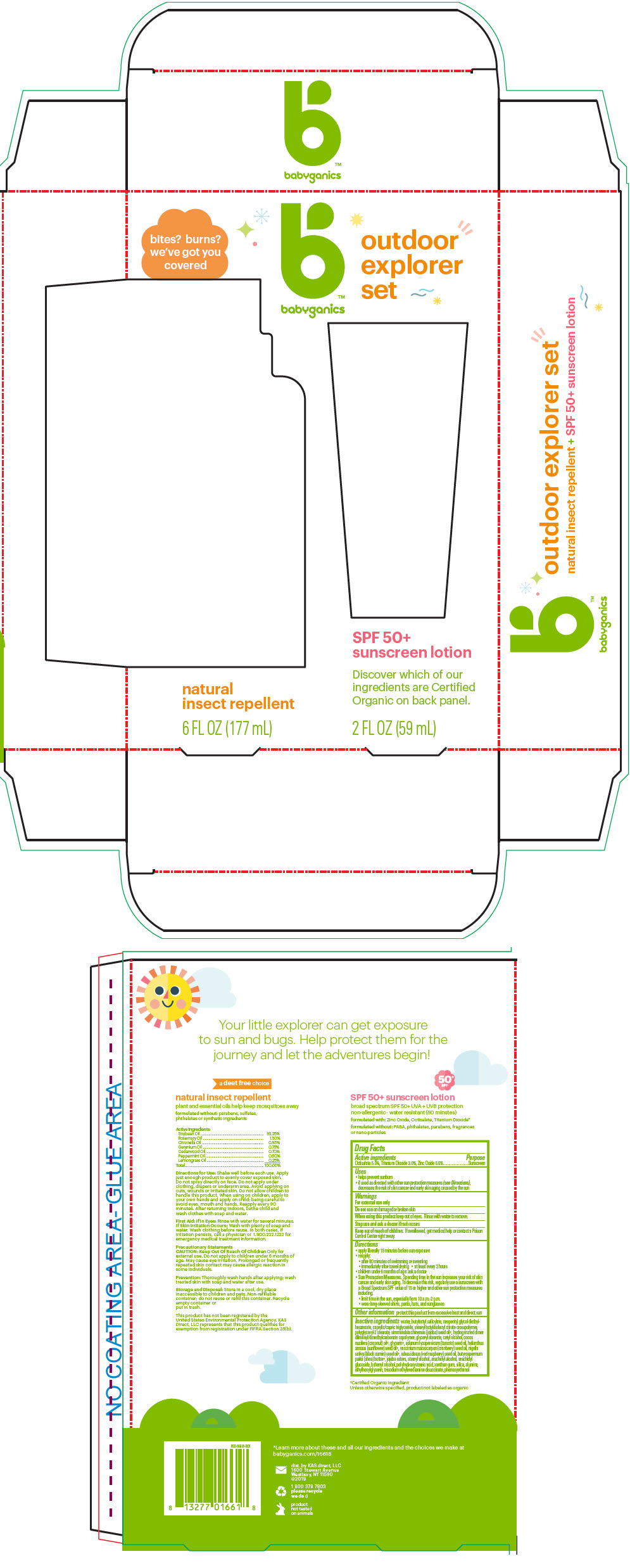
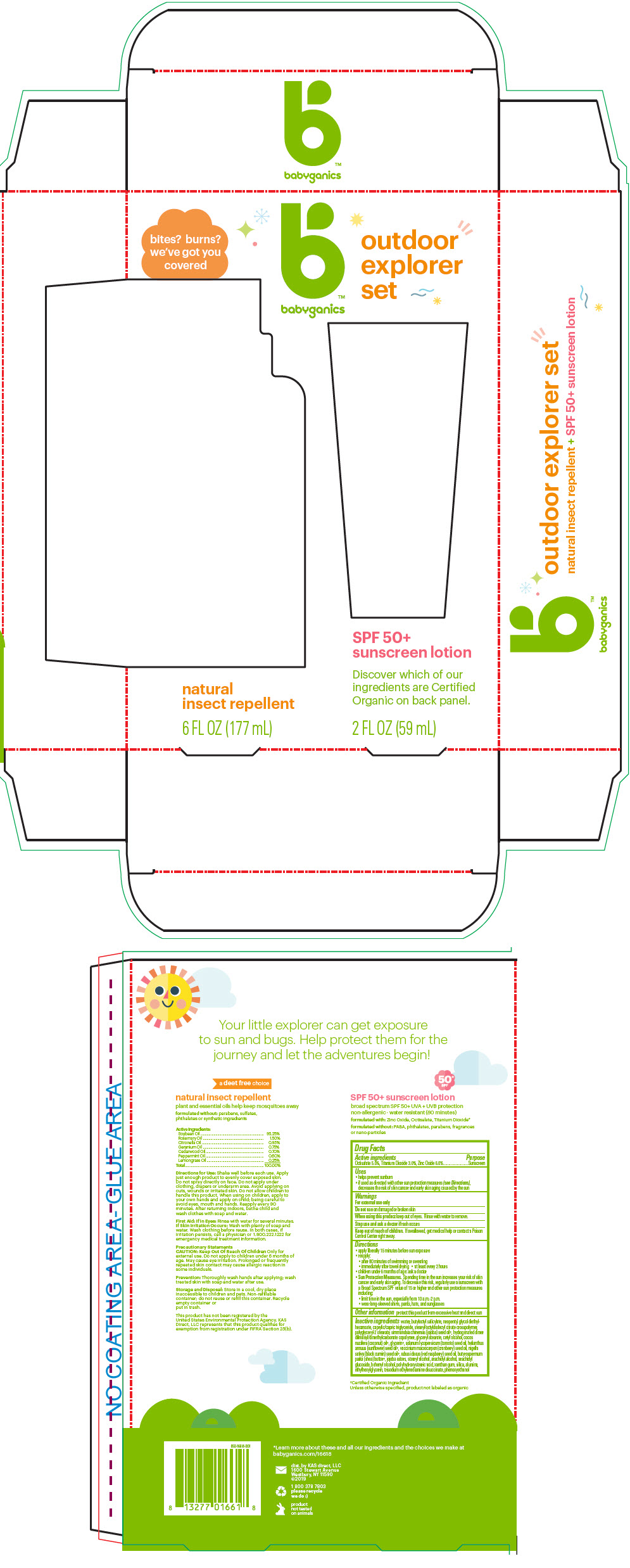
Label: OUTDOOR EXPLORER SET- titanium dioxide, octisalate, and zinc oxide kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 59062-1000-2, 59062-5300-1 - Packager: KAS Direct LLC dba BabyGanics
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 19, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- children under 6 months of age: ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.-2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- Other information
-
Inactive ingredients
water, butyloctyl salicylate, neopentyl glycol diethylhexanoate, caprylic/capric triglyceride, stearyl/octyldodecyl citrate crosspolymer, polyglyceryl-2 stearate, simmondsia chinensis (jojoba) seed oil1, hydrogenated dimer dilinoleyl/dimethylcarbonate copolymer, glyceryl stearate, cetyl alcohol, cocos nucifera (coconut) oil1, glycerin1, solanum lycopersicum (tomato) seed oil, helianthus annuus (sunflower) seed oil1, vaccinium macrocarpon (cranberry) seed oil, nigella sativa (black cumin) seed oil1, rubus idaeus (red raspberry) seed oil, butyrospermum parkii (shea) butter1, jojoba esters, stearyl alcohol, arachidyl alcohol, arachidyl glucoside, behenyl alcohol, polyhydroxystearic acid, xanthan gum, silica, alumina, ethylhexylglycerin, trisodium ethylenediamine disuccinate, phenoxyethanol
- 1
- Certified Organic Ingredient
Unless otherwise specified, product not labeled as organic
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - Kit Package
-
INGREDIENTS AND APPEARANCE
OUTDOOR EXPLORER SET
titanium dioxide, octisalate, and zinc oxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59062-5300 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59062-5300-1 1 in 1 PACKAGE 01/01/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 59 mL Part 2 1 BOTTLE, SPRAY 177 mL Part 1 of 2 SPF 50 PLUS SUNSCREEN
titanium dioxide, octisalate, and zinc oxide lotionProduct Information Item Code (Source) NDC:59062-1000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 30 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 60 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Butyloctyl Salicylate (UNII: 2EH13UN8D3) Neopentyl Glycol Diethylhexanoate (UNII: U68ZV6W62C) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Stearyl/Octyldodecyl Citrate Crosspolymer (UNII: PN88NW0KPK) Polyglyceryl-2 Stearate (UNII: 253MC0P0YV) Cetyl Alcohol (UNII: 936JST6JCN) JOJOBA OIL (UNII: 724GKU717M) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) COCONUT OIL (UNII: Q9L0O73W7L) TOMATO SEED OIL (UNII: 7N87T9C06T) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CRANBERRY SEED OIL (UNII: 73KDS3BW5E) Nigella Sativa Seed Oil (UNII: CS4U38E731) RASPBERRY SEED OIL (UNII: 9S8867952A) PHENOXYETHANOL (UNII: HIE492ZZ3T) XANTHAN GUM (UNII: TTV12P4NEE) SHEA BUTTER (UNII: K49155WL9Y) JOJOBA OIL, RANDOMIZED (UNII: 7F0EV20QYL) Stearyl Alcohol (UNII: 2KR89I4H1Y) Arachidyl Alcohol (UNII: 1QR1QRA9BU) Arachidyl Glucoside (UNII: 6JVW35JOOJ) DOCOSANOL (UNII: 9G1OE216XY) POLYHYDROXYSTEARIC ACID STEARATE (UNII: 8KQ7I65XZE) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ALUMINUM OXIDE (UNII: LMI26O6933) Trisodium Ethylenediamine Disuccinate (UNII: YA22H34H9Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59062-1000-2 59 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 07/24/2017 Part 2 of 2 NATURAL INSECT REPELLENT
lotions, oils, powders, and creams sprayProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR soybean oil (UNII: 241ATL177A) INGR rosemary oil (UNII: 8LGU7VM393) INGR Citronella Oil (UNII: QYO8Q067D0) INGR GERANIUM OIL, ALGERIAN TYPE (UNII: 5Q1I94P4WG) INGR JUNIPERUS VIRGINIANA OIL (UNII: PAD4FN7P2G) INGR Peppermint Oil (UNII: AV092KU4JH) INGR WEST INDIAN LEMONGRASS OIL (UNII: 5BIA40E9ED) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 177 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 01/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/01/2018 Labeler - KAS Direct LLC dba BabyGanics (002764605)