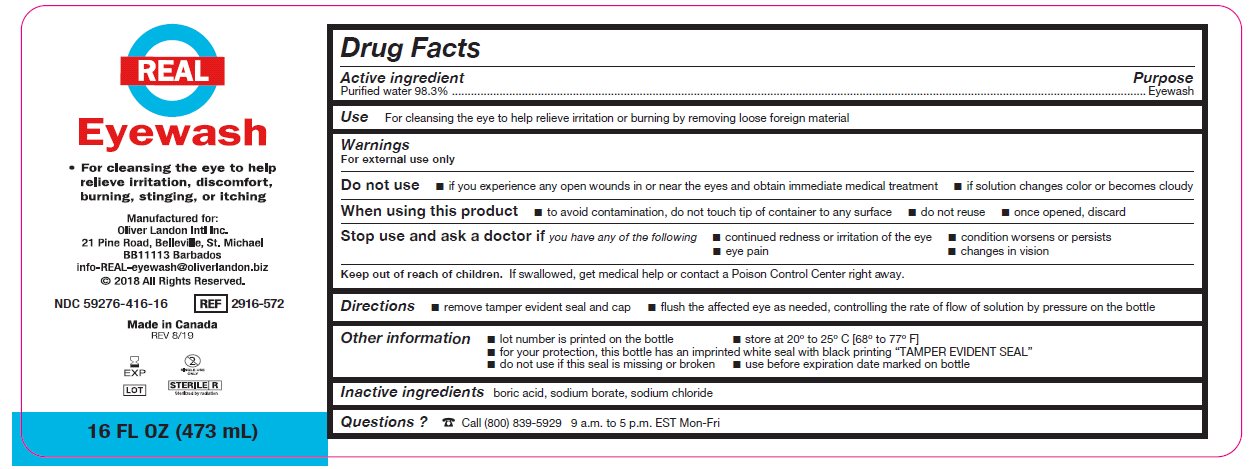
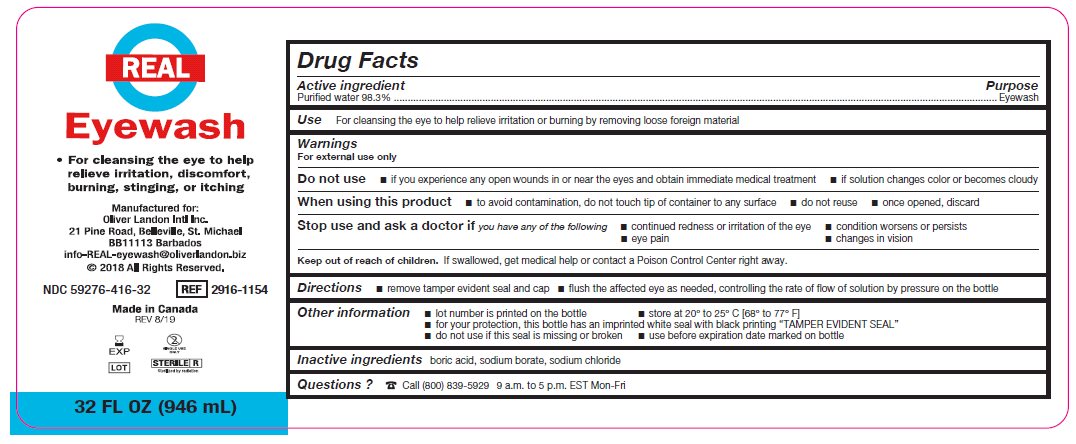
Label: REAL EYEWASH- eyewash solution
-
NDC Code(s):
59276-416-01,
59276-416-04,
59276-416-08,
59276-416-16, view more59276-416-32
- Packager: Oliver Landon Intl Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 22, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
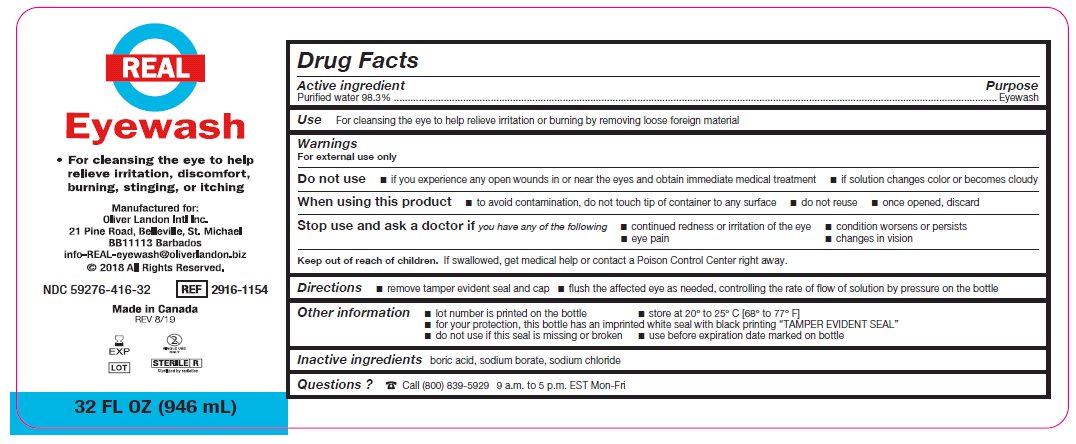
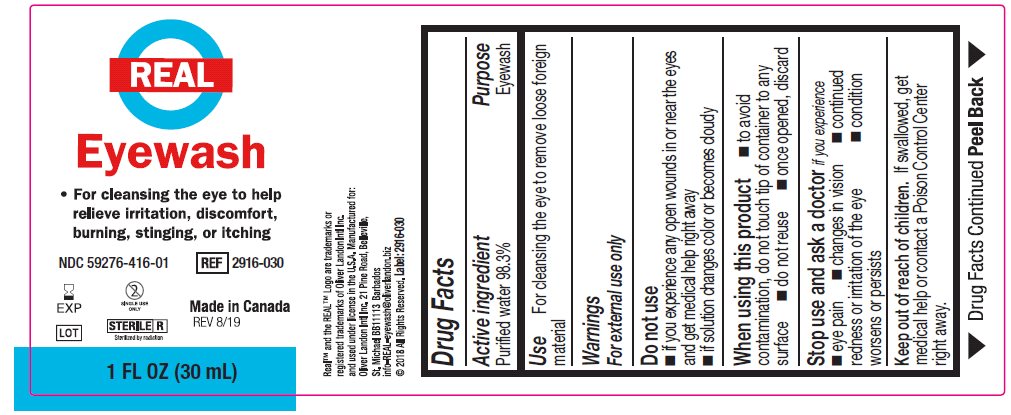
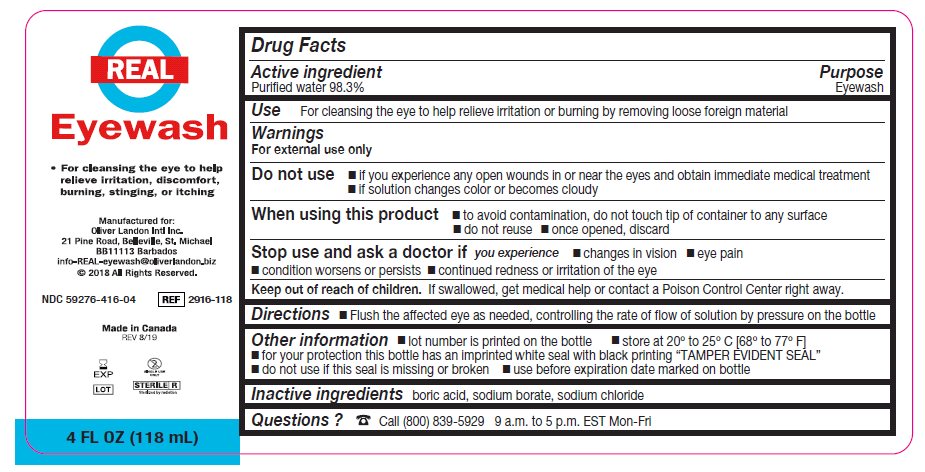
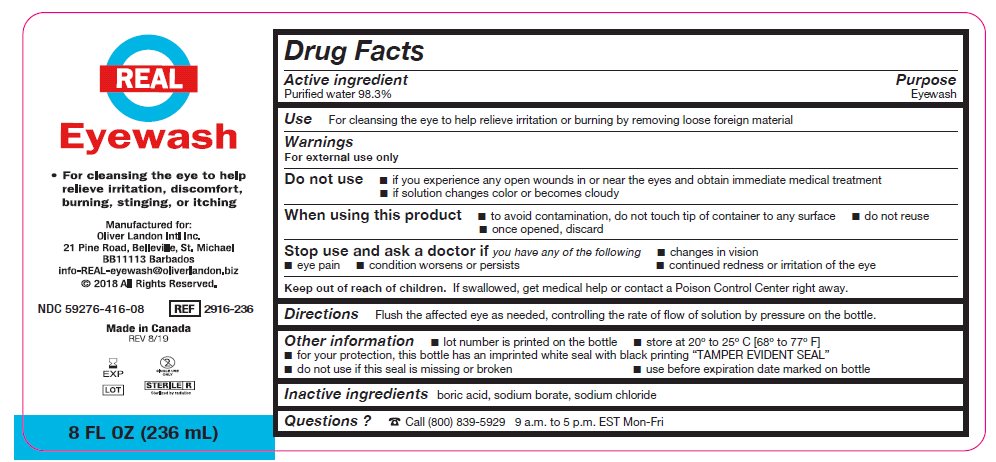
- Active ingredient
- Purpose
-
Warnings
For external use only
Do not use
- •
- if you experience any open wounds in or near the eyes and obtain immediate medical treatment
- •
- if solution changes color or becomes cloudy
When using this product
- •
- to avoid contamination, do not touch tip of container to any surface
- •
- do not reuse
- •
- once opened, discard
Stop use and ask a doctor if you have any of the following
- •
- changes in vision
- •
- eye pain
- •
- condition worsens or persists
- •
- continued redness or irritation of the eye
- Use
- Keep out of reach of children
- Directions
- Other information
- Inactive ingredients
- Questions?
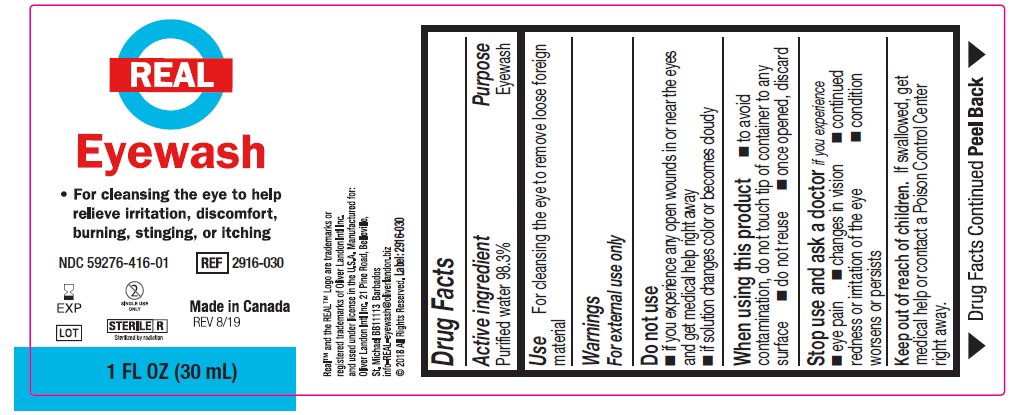
- Package/Label Principal Display Panel – 1 FL OZ (30 mL) label
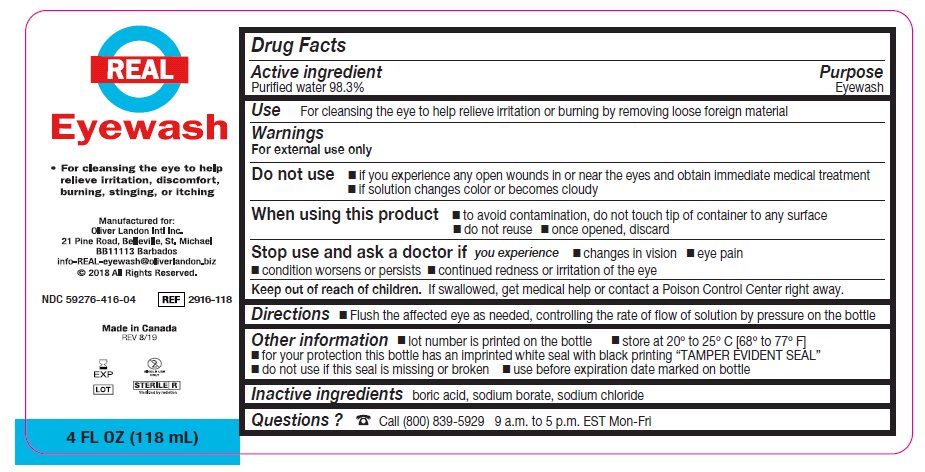
- Package/Label Principal Display Panel – 4 FL OZ (118 mL)
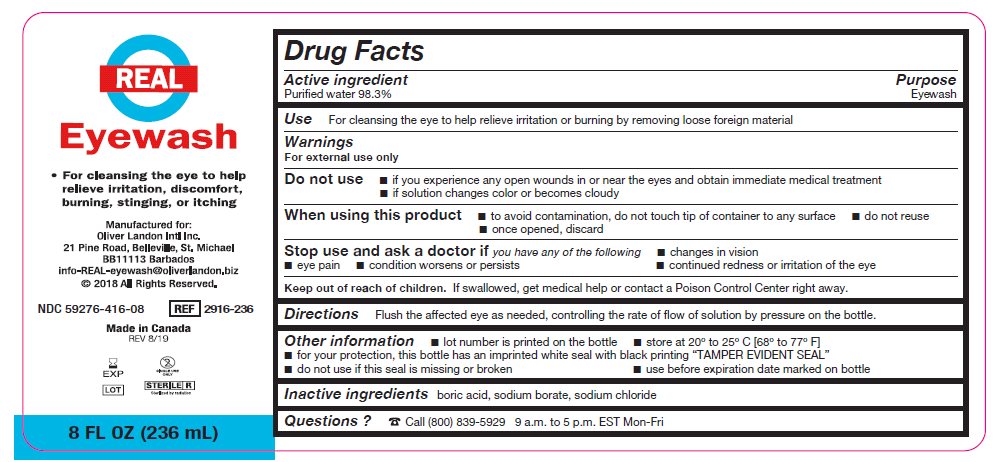
- Package/Label Principal Display Panel – 8 FL OZ (236 mL)
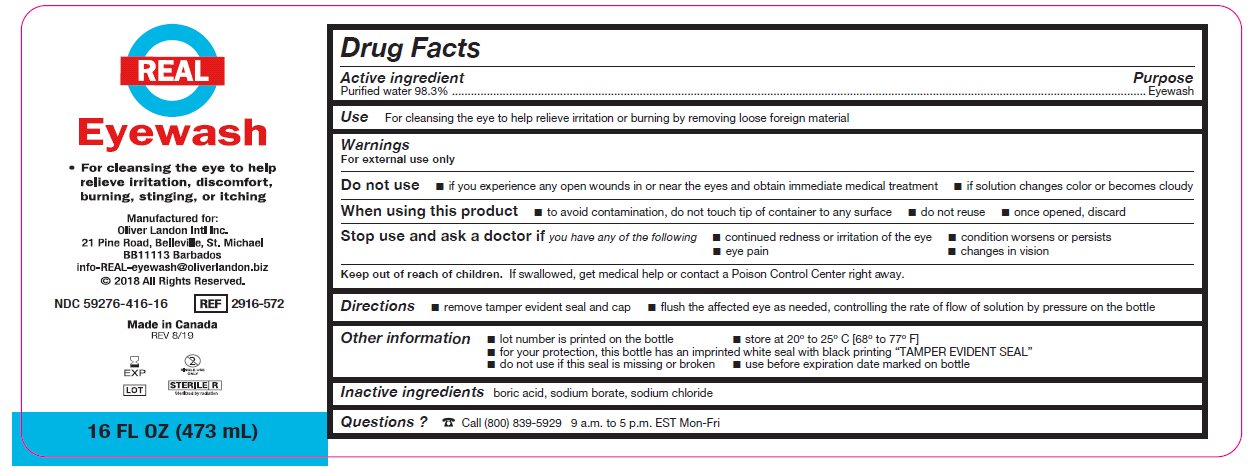
- Package/Label Principal Display Panel – 16 FL OZ (473 mL)
- Package/Label Principal Display Panel – 32 FL OZ (946 mL)
-
INGREDIENTS AND APPEARANCE
REAL EYEWASH
eyewash solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59276-416 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 0.983 mL in 1 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) SODIUM BORATE (UNII: 91MBZ8H3QO) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59276-416-01 30 mL in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 09/01/2015 2 NDC:59276-416-04 118 mL in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 09/01/2015 3 NDC:59276-416-08 236 mL in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 09/01/2015 4 NDC:59276-416-16 473 mL in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 09/01/2015 5 NDC:59276-416-32 946 mL in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 09/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022305 01/30/2015 Labeler - Oliver Landon Intl Inc. (815240195) Registrant - Oliver Landon Intl Inc. (815240195) Establishment Name Address ID/FEI Business Operations Niagara Pharmaceuticals, Inc. 205477792 MANUFACTURE(59276-416)