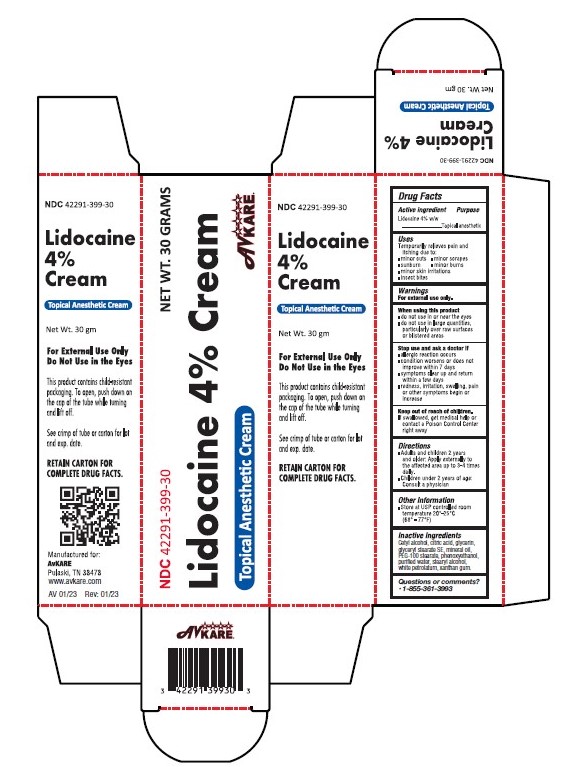
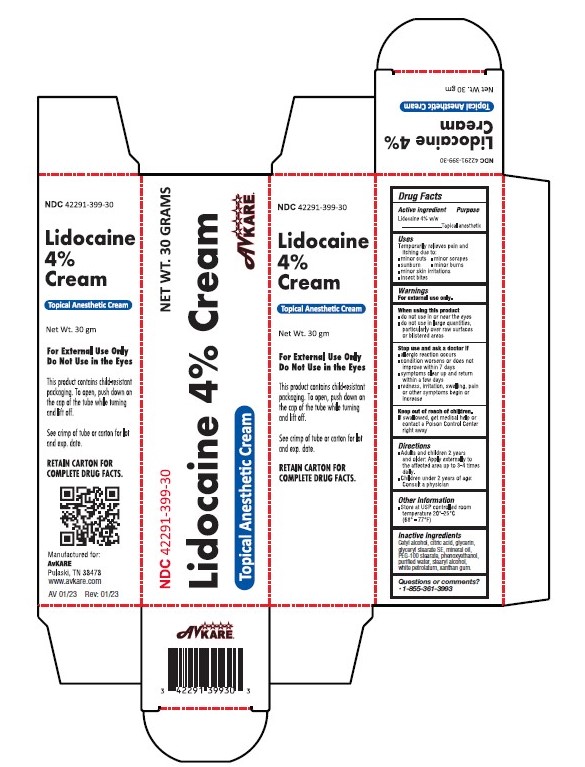
Label: LIDOCAINE- lidocaine 4% cream
- NDC Code(s): 42291-399-30
- Packager: AvKARE
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient:
- Purpose
- Uses
-
WARNINGS
For external use only.
When using this product
- do not use in or near the eyes
- do not use in large quantities, particularly over raw surfaces or blistered areas
- Directions
- Other Information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIDOCAINE
lidocaine 4% creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42291-399 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g Inactive Ingredients Ingredient Name Strength PHENOXYETHANOL (UNII: HIE492ZZ3T) MINERAL OIL (UNII: T5L8T28FGP) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERIN (UNII: PDC6A3C0OX) PEG-100 STEARATE (UNII: YD01N1999R) XANTHAN GUM (UNII: TTV12P4NEE) WATER (UNII: 059QF0KO0R) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) PETROLATUM (UNII: 4T6H12BN9U) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42291-399-30 1 in 1 CARTON 07/10/2018 1 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 07/10/2018 Labeler - AvKARE (796560394)