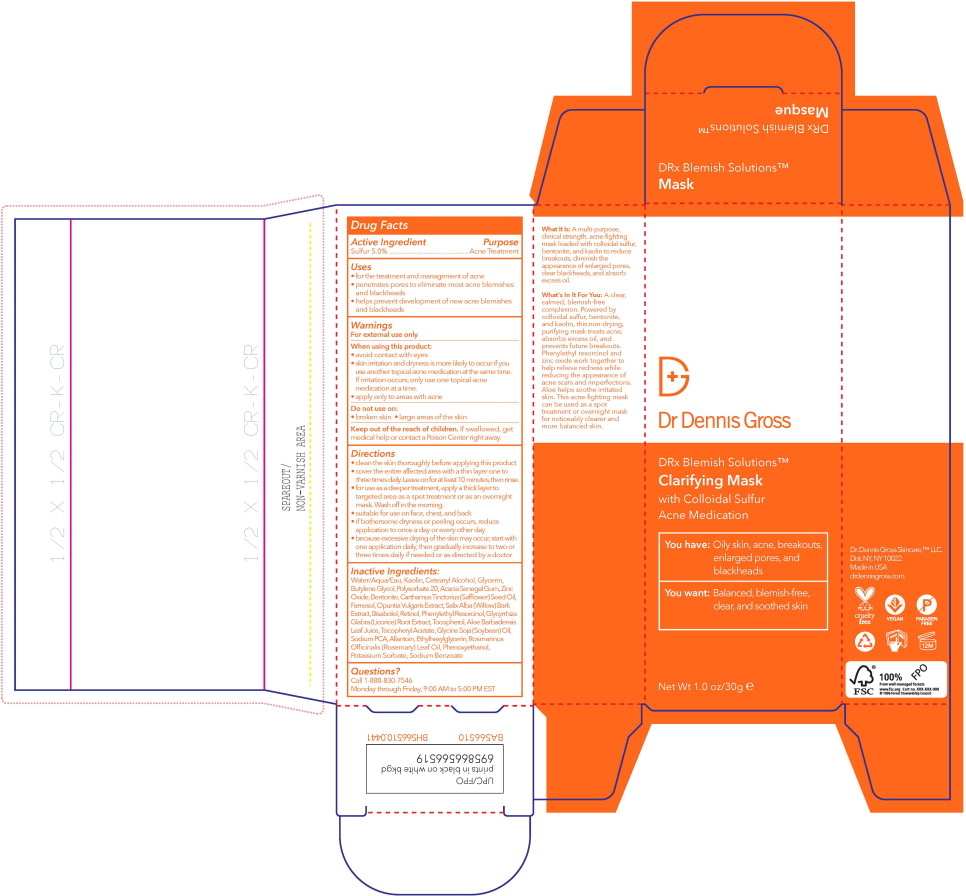
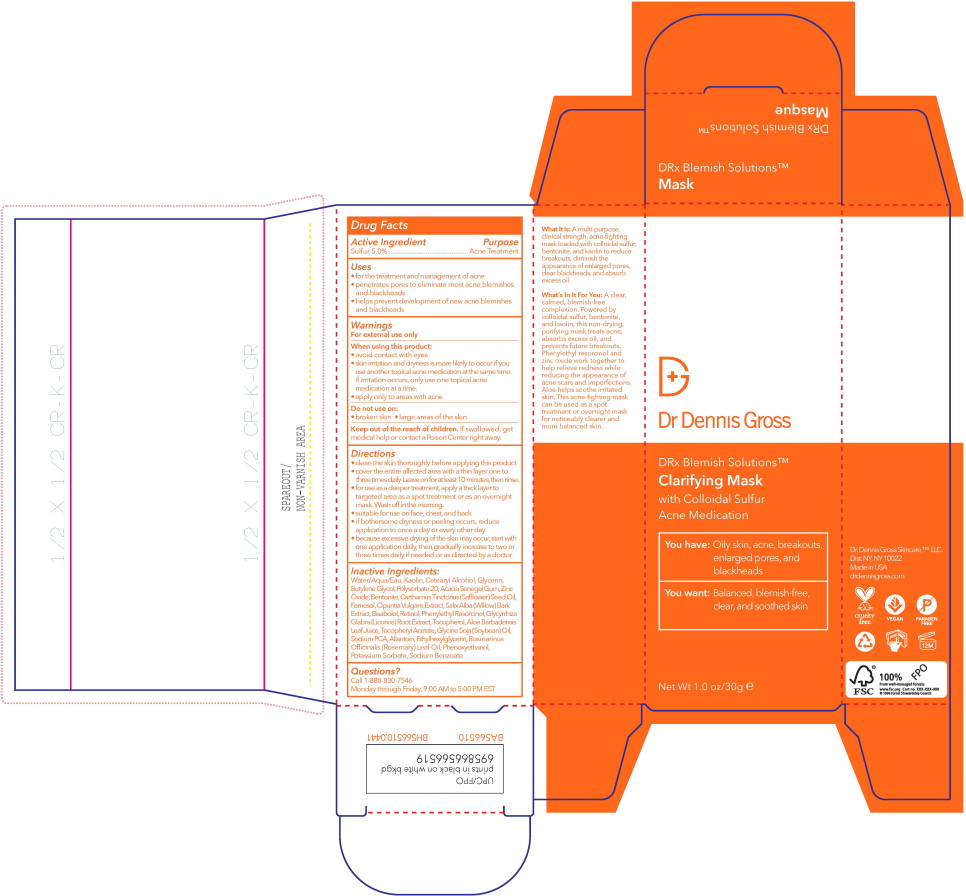
Label: DRX BLEMISH CLARIFYING MASK- sulfur lotion
- NDC Code(s): 58633-704-10
- Packager: Dr. Dennis Gross Skincare
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
-
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily. Leave on for at least 10 minutes, then rinse.
- for use as a deeper treatment, apply a thick layer to targeted area as a spot treatment or as an overnight mask. Wash off in the morning.
- suitable for use on face, chest, and back
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
-
Inactive Ingredients:
Water/Aqua/Eau, Kaolin, Cetearyl Alcohol, Glycerin, Butylene Glycol, Polysorbate 20, Acacia Senegal Gum, Zinc Oxide, Bentonite, Carthamus Tinctorius (Safflower) Seed Oil, Farnesol, Opuntia Vulgaris Extract, Salix Alba (Willow) Bark Extract, Bisabolol, Retinol, Phenylethyl Resorcinol, Glycyrrhiza Glabra (Licorice) Root Extract, Tocopherol, Aloe Barbadensis LeafJuice, Tocopheryl Acetate, Glycine Soja (Soybean) Oil, Sodium PCA, Allantoin, Ethylhexylglycerin, Rosmarinus Officinalis (Rosemary) Leaf Oil, Phenoxyethanol, Potassium Sorbate, Sodium Benzoate
- Questions?
- Principal Display Panel – 30 mL Box Label
-
INGREDIENTS AND APPEARANCE
DRX BLEMISH CLARIFYING MASK
sulfur lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58633-704 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sulfur (UNII: 70FD1KFU70) (Sulfur - UNII:70FD1KFU70) Sulfur 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Kaolin (UNII: 24H4NWX5CO) Cetostearyl Alcohol (UNII: 2DMT128M1S) Butylene Glycol (UNII: 3XUS85K0RA) Glycerin (UNII: PDC6A3C0OX) Polysorbate 60 (UNII: CAL22UVI4M) Acacia (UNII: 5C5403N26O) Zinc Oxide (UNII: SOI2LOH54Z) Bentonite (UNII: A3N5ZCN45C) Safflower Oil (UNII: 65UEH262IS) Phenoxyethanol (UNII: HIE492ZZ3T) Levomenol (UNII: 24WE03BX2T) Allantoin (UNII: 344S277G0Z) Rosemary Oil (UNII: 8LGU7VM393) Aloe Vera Leaf (UNII: ZY81Z83H0X) Phenylethyl Resorcinol (UNII: G37UFG162O) Sodium Pyrrolidone Carboxylate (UNII: 469OTG57A2) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Ethylhexylglycerin (UNII: 147D247K3P) Soybean Oil (UNII: 241ATL177A) Opuntia Ficus-Indica Whole (UNII: 23Z87HTQ6P) Farnesol (UNII: EB41QIU6JL) Glycyrrhiza Glabra (UNII: 2788Z9758H) Salix Alba Bark (UNII: 205MXS71H7) Retinol (UNII: G2SH0XKK91) Citric Acid Monohydrate (UNII: 2968PHW8QP) Potassium Sorbate (UNII: 1VPU26JZZ4) Sodium Benzoate (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58633-704-10 1 in 1 BOX 03/01/2020 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 03/01/2020 Labeler - Dr. Dennis Gross Skincare (008190808)