Label: NITROUS OXIDE gas
-
Contains inactivated NDC Code(s)
NDC Code(s): 72223-001-01 - Packager: Praxair Mexico, S. de R.L. de C.V
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 9, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
NITROUS OXIDE USP LABEL
PRAXAIR Praxair Mexico S. de, R.L. de C.V.
Laboraicarlo de Costrel de Calidad de Producto
Plasia Toluca
Av Independence Ote No 300
CP 50070 Toluca Ede de Mexico
Tel (722)2358800
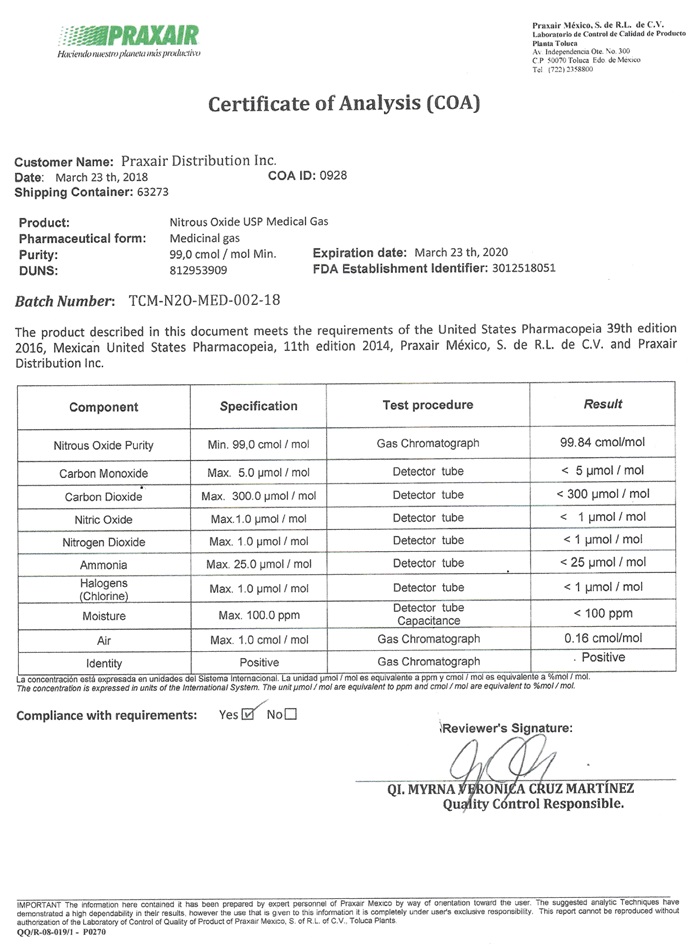
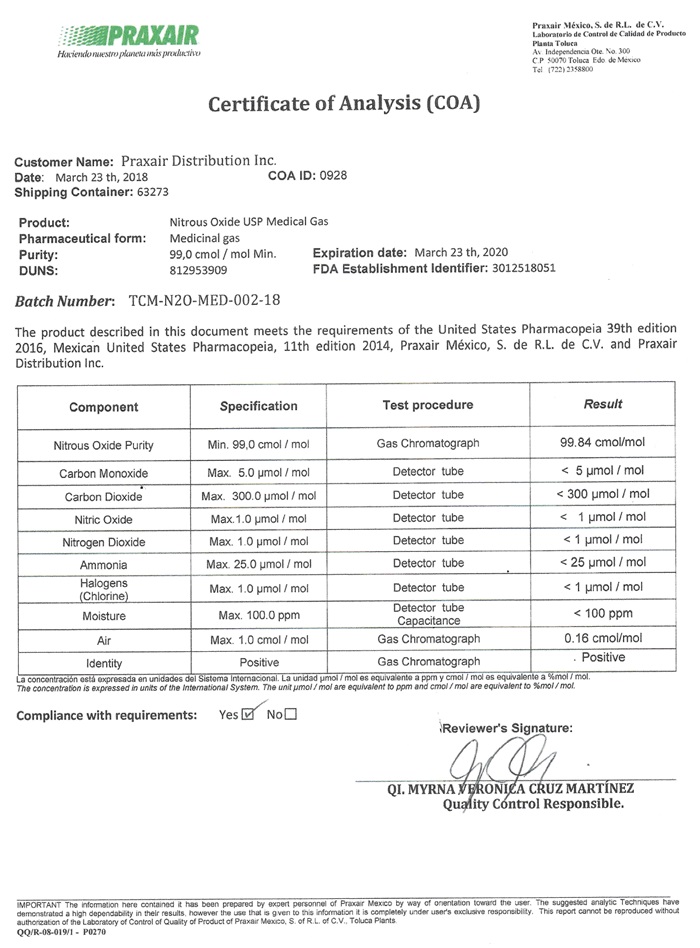
Certificate of Analysis (COA)
Customer Name: Praxair Distribution Inc.
Date: March 23 th, 2018 COA US 0928
Shipping Container: 63273
Product: Nitrous Oxide USP Medical Gas
Pharmaceutical form: Medicinal gas
Purity: 99.0 cmol/ mol Min. Expiration Date: March 23 th, 2020
DUNS: 8129539029 FDA Establishment Identifier: 3012518051
Batch Number: TCM-N20-MED-002-18
This product described in this document meets the requirements of the United States Pharmacopeia 39th edition 2016, Mexican United States Pharmacopeia, 11th edition 2014, Praxair Mexico, S. de R.L. de C.V. and Praxair Distribution, Inc.
Component Specification Test Procedure Results
Nitrous Oxide Purity Min. 99.0 cmol/moi Gas Chromatograph 99.84 cmol/mol
Carbon Monoxide Max. 5.0 µmol/mol Detector tube < 5 µmol/mol
Carbon Dioxide Max. 300.0 µmol/mol Detector tube < 300 µmol/mol
Nitric Oxide Max. 1.0 µmol/mol Detector tube < 1 µmol/mol
Nitrogen Dioxide Max. 1.0 µmol/mol Detector tube < 1 µmol/mol
Ammonia Max. 25.0 µmol/mol Detector tube < 25 µmol/mol
Halogens
(Chlorine) Max. 1.0 µmol/mol Detector tube < 1 µmol/mol
Moisture Max. 100.0 ppm Detector tube < 100 ppm
Capacitance
Air Max 1.0 cmol/mol Gas Chromatograph 0.16 cmol/mol
Identity Positive Gas Chromatograph Positive
La concentracion esto expresada en unidades del Sistoma Internaciona La unidad µmol/mol
Es equivalente a porm y cmol/ mol es equivalente a %mol/mol.
The concentration is expressed in units of the International System. The unit µmol/mol are equivalent to ppm and cmol/mol are equivalent to % mol/mol.
Compliance with requirements: Yes _X_ No ___
Reviewer’s Signature:
____________________________________________
QI. MYRNA VEONICA CRUZ MARTINEZ
Quality Control Responsible.
IMPORTANT The information here contained it has been prepared by expert personnel of Praxair Mexico by way of orientation toward the user. The suggested analytic Techniques have demonstrated a high dependability I their results, however, the use that is given to the information it is completely user user’s exclusive responsibility. This report cannot be reportability. This report cannot be reproduced without authorization of the Laboratory of Coontrol of Quality of Product of Praxair Mexidom, S. de, R.L. de C.V., Toluca Plants.
QQ/r-08-019/1 – P0270
-
INGREDIENTS AND APPEARANCE
NITROUS OXIDE
nitrous oxide gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72223-001 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITROUS OXIDE (UNII: K50XQU1029) (NITROUS OXIDE - UNII:K50XQU1029) NITROUS OXIDE 990 mL in 1 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72223-001-01 7064200 L in 1 CONTAINER; Type 0: Not a Combination Product 06/08/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210235 06/08/2017 Labeler - Praxair Mexico, S. de R.L. de C.V (812953909) Establishment Name Address ID/FEI Business Operations Praxair Mexico, S. de R.L. de C.V 812953909 manufacture(72223-001) Establishment Name Address ID/FEI Business Operations Praxair Mexico, S. de R.L. de C.V 812821245 manufacture(72223-001)