Label: GUAIFENESIN tablet, extended release
-
NDC Code(s):
51660-566-21,
51660-566-41,
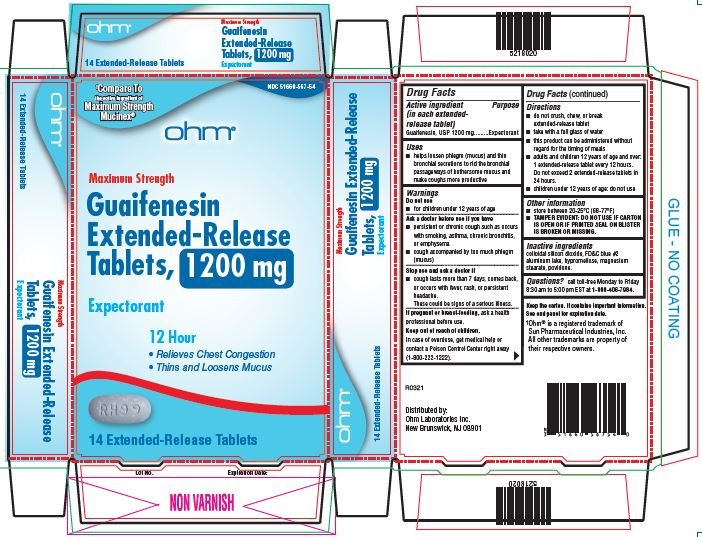
51660-567-54,
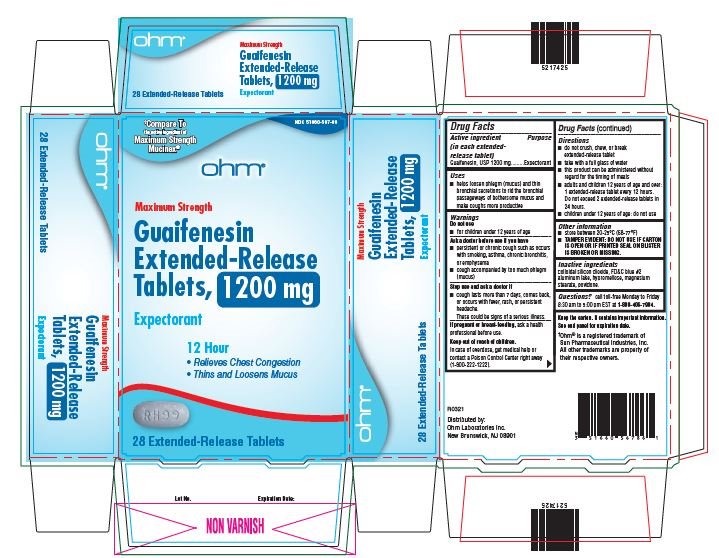
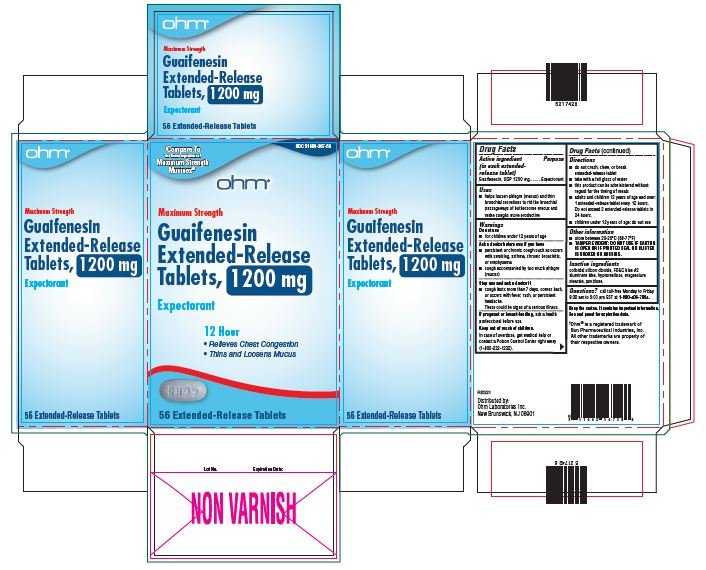
51660-567-58, view more51660-567-86
- Packager: Ohm Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
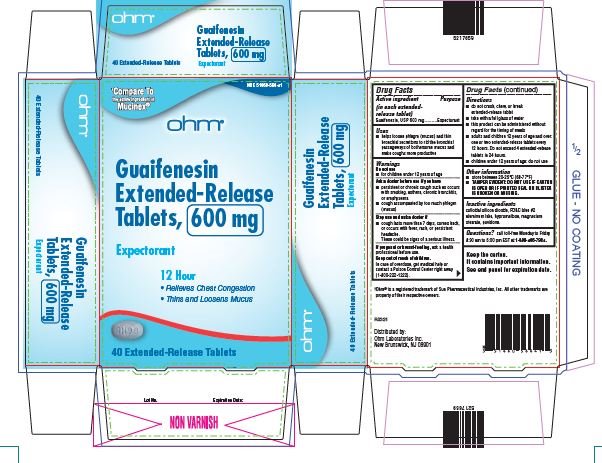
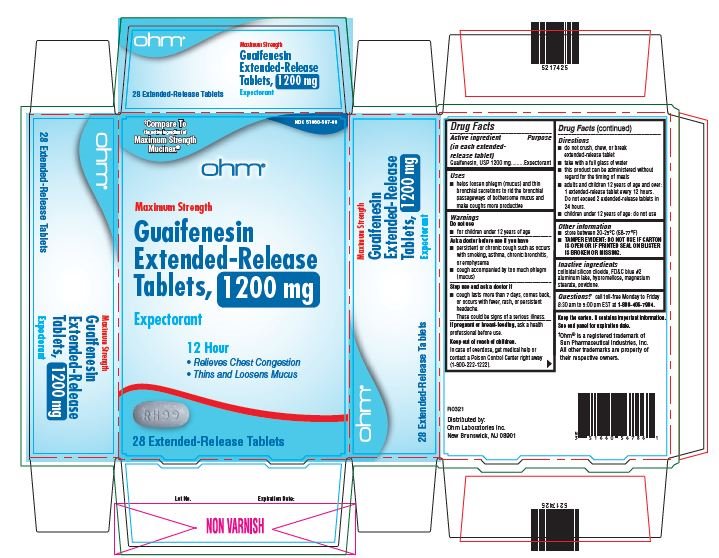
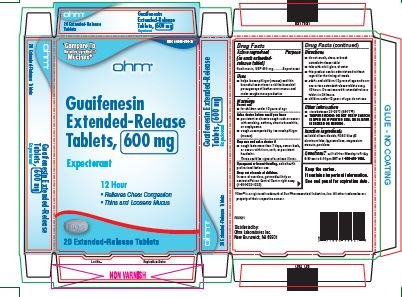
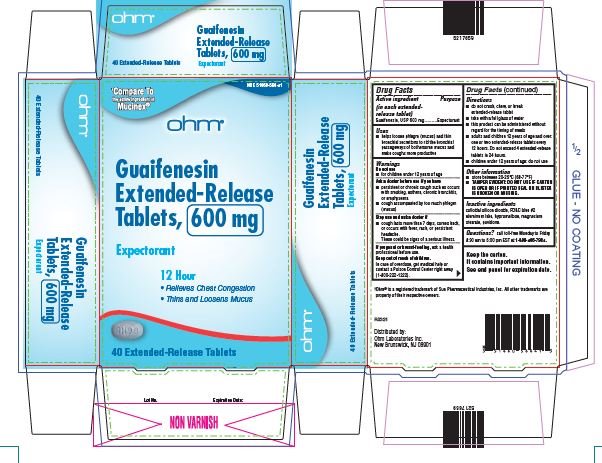
- Active ingredient
- Purpose
- Keep Out of Reach of Children
- Uses
-
Warnings
Do not use
- •
- for children under 12 years of age
Ask a doctor before use if you have
- •
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- •
- cough accompanied by too much phlegm (mucus)
Stop use and ask a doctor if
- •
- cough lasts more than 7 days, comes back or occurs with fever, rash, or persistent headache.
- These could be signs of serious illness.
If pregnant or breast-feeding, ask a health professional before use.
-
Directions
- •
- do not crush, chew or break extended-release tablet
- •
- take with a full glass of water
- •
- this product can be administered without regard for the timing of meals
- •
- adults and children over 12 years of age and over: one or two extended-release tablets every 12 hours. Do not exceed 4 extended-release tablets in 24 hours.
- •
- children under 12 years of age: do not use.
Other information
- •
- store between 20-25°C (68-77°F)
- •
- TAMPER EVIDENT: DO NOT USE IF CARTON IS OPEN OR IF PRINTED SEAL ON BLISTER IS BROKEN OR MISSING.
Keep the carton. It contains important information. See end panel for expiration date.
†Ohm® is a registered trademark of Sun Pharmaceutical Industries, Inc. All other trademarks are property of their respective owners.
- Inactive Ingredients
- Package/Label Principal Display Panel
- Package/Label Principal Display Panel
- Principal Display Panel
- Principal Display Panel
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN
guaifenesin tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51660-566 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 600 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) Product Characteristics Color WHITE (blue/white mottled) Score no score Shape OVAL Size 16mm Flavor Imprint Code RH;98 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51660-566-41 40 in 1 CARTON; Type 0: Not a Combination Product 04/01/2022 2 NDC:51660-566-21 20 in 1 CARTON; Type 0: Not a Combination Product 04/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209254 04/01/2022 GUAIFENESIN
guaifenesin tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51660-567 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 1200 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) Product Characteristics Color WHITE (blue/white mottled) Score no score Shape OVAL Size 16mm Flavor Imprint Code RH;99 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51660-567-54 14 in 1 CARTON; Type 0: Not a Combination Product 04/01/2022 2 NDC:51660-567-86 28 in 1 CARTON; Type 0: Not a Combination Product 04/01/2022 3 NDC:51660-567-58 56 in 1 CARTON; Type 0: Not a Combination Product 04/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209254 04/01/2022 Labeler - Ohm Laboratories, Inc. (184769029) Establishment Name Address ID/FEI Business Operations Ohm Laboratories, Inc. 184769029 MANUFACTURE(51660-566, 51660-567) , ANALYSIS(51660-566, 51660-567) , PACK(51660-566, 51660-567) Establishment Name Address ID/FEI Business Operations Sun Pharmaceutical Industries Limited 650456002 MANUFACTURE(51660-566, 51660-567)