Label: VITALIPID N- vitamin a palmitate, ergocalciferol, .alpha.-tocopherol, dl-, phytonadione emulsion
-
NDC Code(s):
65219-335-10,
65219-335-82,
65219-337-10,
65219-337-62, view more65219-339-10, 65219-339-64
- Packager: Fresenius Kabi USA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Unapproved drug for use in drug shortage
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HEALTH CARE PROVIDER LETTER
Fresenius Kabi USA, LLC
Three Corporate Drive
Lake Zurich, Illinois 60047
T 847-550-2300
T 888-391-6300September 1, 2023 www.fresenius-kabi.us IMPORTANT PRESCRIBING INFORMATION Subject: Temporary importation of Vitalipid N ADULT and Vitalipid N INFANT to address drug shortage. The product is also labeled as Vitlipid N ADULT and Vitlipid N INFANT. Dear Healthcare Professional,
Due to the critical shortage of parenteral multivitamins in the U.S. market, Fresenius Kabi USA, LLC (Fresenius Kabi USA) is coordinating with the U.S. Food and Drug Administration (FDA) to provide an alternative treatment option during this time. Fresenius Kabi USA has initiated temporary importation of Vitalipid Injection 10 mL Single Dose Glass Ampule into the U.S. market. The products imported into the U.S. may also be labeled with a slight variation of the proprietary name, Vitlipid N ADULT and Vitlipid N INFANT, depending on which country the product was originally intended. Other than the slight variation in the proprietary name, the products are identical, and the products will be referred to as Vitalipid N ADULT and Vitalipid N INFANT for the remainder of this letter. Vitalipid N ADULT and Vitalipid N INFANT are sterile oil-in-water emulsions containing fat-soluble vitamins in the oil phase. This product is marketed in Europe and is manufactured in the Fresenius Kabi Uppsala, Sweden plant.
At this time, no other entity except Fresenius Kabi USA is authorized by the FDA to import or distribute Vitalipid Injection 10 mL Single Dose Glass Ampules (One Point Cut) in the U.S. The FDA has not approved Fresenius Kabi's Vitalipid product for marketing in the United States.
This communication and product information is available on the Fresenius Kabi USA web site http://products.fresenius-kabi.us/product-323.html as well as on the FDA Drug Shortage web site. http://www.fda.gov/Drugs/DrugSafety/DrugShortages/default.htm.
IMPORTANT DRUG INFORMATION
It is important to note the following key differences between the Vitalipid and Infuvite:
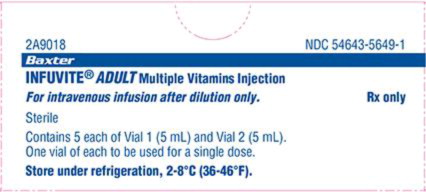
Product Name U.S. FDA-Approved Product (Infuvite Adult)1 Imported Product (Vitalipid N Adult)2,3 U.S. FDA-Approved Product (Infuvite Pediatric)4 Imported Product (Vitalipid N Infant)5,6 Manufacturer Baxter Fresenius Kabi Baxter Fresenius Kabi Indication For prevention of vitamin deficiency in adults and children aged 11 and older receiving parenteral nutrition Indicated in adult patients and children from 11 years of age as a supplement in intravenous nutrition to meet the daily requirements of the fat-soluble vitamins A, D2, E and K1. For the prevention of vitamin deficiency in pediatric patients up to 11 years of age receiving parenteral nutrition Indicated in infants and children up to 11 years of age as a supplement in intravenous nutrition to meet the daily requirements of the fat-soluble vitamins A, D2, E and K1. Product ingredients present 13 vitamins present (water and fat soluble)
Only 4 fat-soluble vitamins 13 vitamins present (water and fat soluble) Only 4 fat-soluble vitamins Contraindications Existing hypervitaminosis or history of hypersensitivity due to any vitamins or excipients contained in the formulation Egg, soy, peanut allergy, hypersensitivity to ingredient/excipient Existing hypervitaminosis or history of hypersensitivity due to any vitamins or excipients contained in the formulation Egg, soy, peanut allergy, hypersensitivity to ingredient/excipient Storage Minimize exposure to light because vitamins A, D and riboflavin are light sensitive. Store under refrigeration, 2-8°C (36-46°F). Between 2-25°C (36-77°F). Protect from light. Do not freeze Minimize exposure to light because vitamins A, D and riboflavin are light sensitive. Store under refrigeration, 2-8°C (36-46°F). Between 2-25°C (36-77°F). Protect from light. Do not freeze Bar code/NDC 54643-5649-1 NDC (Finished Product): 6521933381; 6521933582
NDC (Unit of Use): 6521933310; 652193351054643-5646-1 NDC (Finished Product): 6521933762; 6521933964
NDC (Unit of Use): 6521933710; 6521933910- Vitalipid is packaged in glass ampules, so filter needles must always be used in conjunction with a regular (nonfilter) needle during compounding to reduce the risk of introducing glass particles during administration
- Vitalipid provides fat-soluble vitamins (A, D, E, K) only; Infuvite contains fat-soluble vitamins as well as 9 water-soluble vitamins (see Product Comparison Table below)
- Vitalipid N ADULT and INFANT are contraindicated in patients with known hypersensitivity to any of the components (i.e., egg, soy, peanut protein, or any active substance or excipient)
- Any barcodes on the Vitalipid N ADULT or Vitalipid N INFANT products will not be appropriately recognized by scanning systems used in the United States and should NOT be used. Institutions should manually input the product into their systems to confirm that barcode systems do not provide incorrect information when the product is scanned. Alternative procedures should be followed to assure that the correct drug product is being prepared and administered to individual patients.
- Vitalipid N ADULT and Vitalipid N INFANT are available only by prescription in the U.S. However, the imported lots do not have the statement “Rx only” on their labeling.
Effective immediately, and during this temporary period, Fresenius Kabi USA will offer the following presentation of adult and pediatric multivitamins:
Product Description
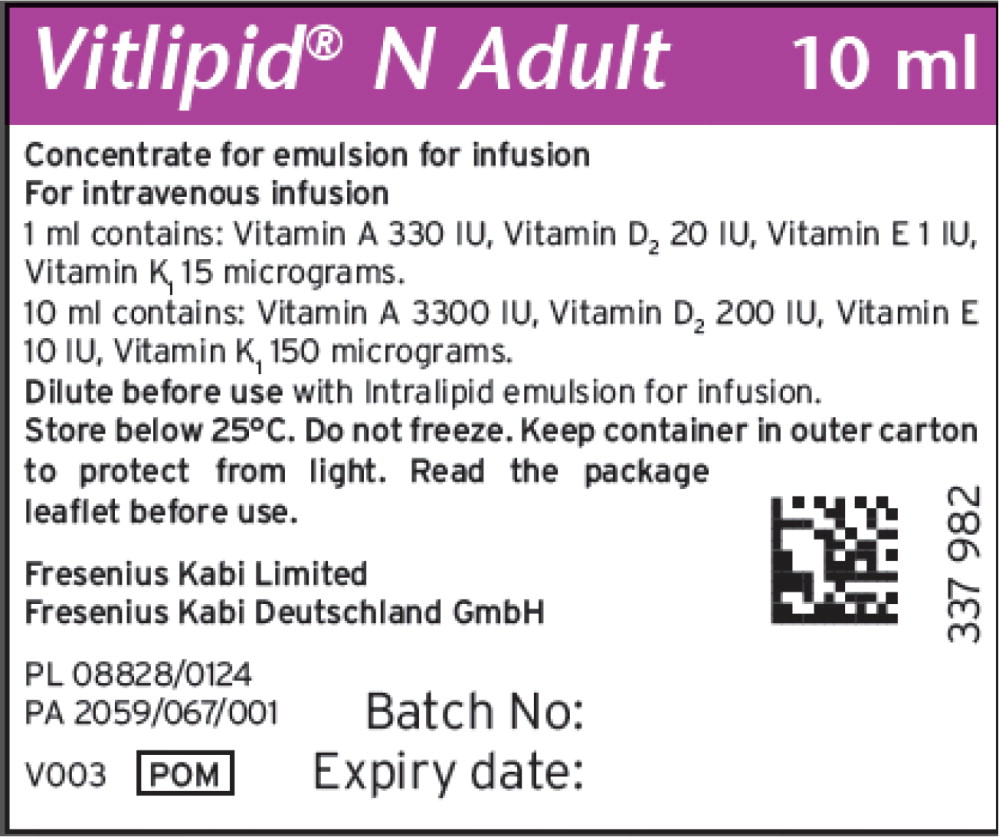
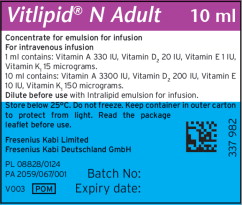
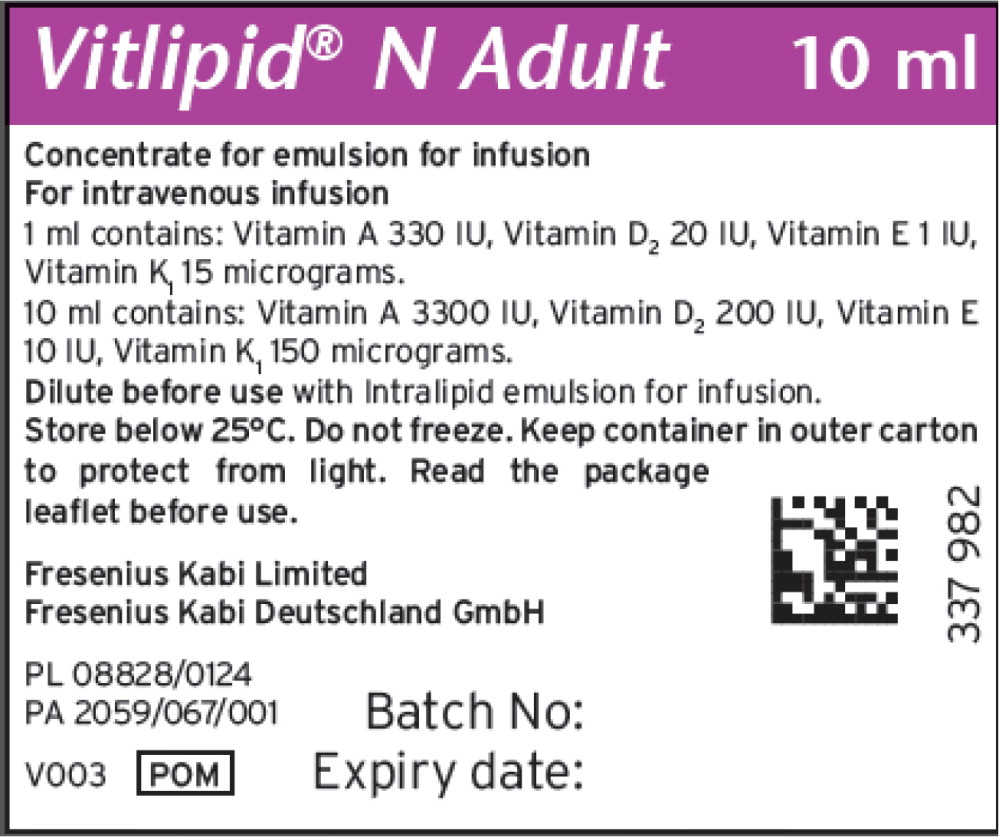
Vitalipid/Vitlipid N ADULT 10 mL2,3
Description Concentrated Emulsion for Injection Dosage Form: 10 mL Single Dose Glass Ampule Package size Box contains 10 units of 10 mL Finished Product NDC 6521933582 Unit of Use NDC 6521933510 Lot Numbers/(Expiration Date) 10SC1307/(2025.02.28); 10SD2190 (2025.03.31) Vitamin Active Ingredients
(per 1 mL)Active Ingredients
(per 10 mL)Retinol palmitate (Vitamin A) 99 mcg (330 IU) 990 mcg (3300 IU) Ergocalciferol (Vitamin D2) 0.5 mcg (20 IU) 5 mcg (200 IU) All-rac-alpha-tocopherol (Vitamin E) 0.91 mg (1IU) 9.1 mg (10 IU) Phytomenadione (Vitamin K1) 15 mcg 150 mcg Excipients (per 1 mL) Excipients (per 10 mL) Purified egg phospholipids 12 mg 120 mg Glycerol anhydrous 22 mg 220 mg Purified soybean oil 100 mg 1000 mg Sodium hydroxide N/A To pH 8 Water for injection N/A To 1 mL Vitalipid/Vitlipid N INFANT 10 mL5,6
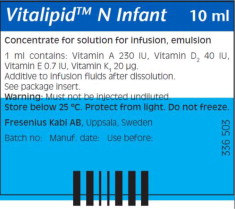
Description Concentrated Emulsion for Injection
Dosage Form: Single Dose Glass Ampule Package Size: Box contains 10 units of 10 mL Finished Product NDC: 6521933762; 6521933964 Unit of Use NDC: 6521933710; 6521933910 Lot Numbers/(Expiration Date) 10SB9030/(2025.01.31); 10SB9028/(2025.03.31) Vitamin Active Ingredients
(per 1 mL)Active Ingredients
(per 10 mL)Retinol palmitate (Vitamin A) 69 mcg (230 IU) 690 mcg (2300 IU) Ergocalciferol (Vitamin D2) 1 mcg (40 IU) 10 mcg (400 IU) All-rac-alpha-tocopherol (Vitamin E) 0.64 mg (0.7 IU) 6.4 mg (7 IU) Phytomenadione (Vitamin K1) 20 mcg 200 mcg Excipients (per 1 mL) Excipients (per 10 mL) Purified egg phospholipids 12 mg 120 mg Glycerol anhydrous 22 mg 220 mg Purified soybean oil 100 mg 1000 mg Sodium hydroxide N/A To pH 8 Water for injection N/A To 1 mL Preparation/Administration
- Vitalipid N ADULT must be diluted before use and strict aseptic technique must always be maintained during handling. Because Vitalipid N ADULT is packaged in a glass ampule, a filter needle must always be used in conjunction with a regular (nonfilter) needle during compounding to reduce the risk of introducing glass particulates during administration.
- Both Vitalipid N ADULT and Vitalipid N INFANT are packaged in One Point Cut (OPC) ampules. OPC ampules are manufactured with a colored dot on the bulbous part of the ampule indicating the position where the ampule should be broken. Please follow the recommended instructions below to open the OPC ampule easily and safely:
- Before an ampule is opened, any solution visible in the top portion (i.e., the head) should be moved to the bottom (i.e., body) by swirling the ampule in an upright position, tapping the ampule with one's finger, or inverting the ampule and then quickly swinging it into an upright position.
- To open an ampule properly, the head should be cleansed with an alcohol swab. The alcohol swab can be left in place or a new alcohol-soaked gauze pad can be applied to prevent accidental cuts to the fingers as well as shattering of glass particles and aerosolized drug.
- Pick up the ampule and hold its lower part (body) between your thumb and index finger and position the ampule so that the colored dot faces you.
- Grasp the top of the ampule with your other hand. Place your thumb over the colored dot and index finger on the opposite side (back) of the bulbous part of the ampule.
- Hold the bottom of the ampule firmly in an upright position and push away from oneself in a quick motion to “snap” open the ampule at the neck.
- Ampules should NOT be opened toward the HEPA filter in the compounding work area (to prevent spraying of liquid) or toward other sterile preparation within the hood.
- A regular needle should be used to withdraw 10 mL from one ampule. The regular needle should be removed from the syringe and replaced with a new filter needle to inject the 10 mL of Vitalipid into 500 mL of Intralipid (Fresenius Kabi). To ensure a homogeneous admixture, the bag should be inverted a couple of times immediately before the infusion. Vitalipid N ADULT is also used as a complement in parenteral nutrition (PN) formulations. Questions about compatibility may be directed to Fresenius Kabi USA, LLC Medical Affairs.
- Vitalipid N ADULT should be added to an infusion within one hour of administration and the infusion should be completed within 24 hours from preparation to prevent microbiological contamination. Any remaining contents of opened ampules should be discarded.
- Vitalipid N ADULT has been tested in PN formulations available outside of the U.S. containing lipid injectable emulsions under European standards and demonstrated compatibility with non-U.S. approved amino acids and other PN products. Branded U.S. PN products tested that have shown compatibility are SMOFlipid and Kabiven. Similar tests have not been conducted using U.S. amino acid products, thus caution should be taken when adding Vitalipid N ADULT to adult PN formulations. Vitalipid has also demonstrated compatibility when added to Kabiven in combination with some US amino acid branded products (e.g., Travasol 10% [Baxter Healthcare], Aminosyn II 15% [Hospira, Inc], Clinisol 15% [Baxter Healthcare], Prosol 20% [Baxter Healthcare]).
- Vitalipid N INFANT must be diluted before use in Intralipid 20% (Fresenius Kabi). The daily dose must not exceed 10 mL. Because Vitalipid N INFANT is packaged in a glass ampule, a filter needle must always be used in conjunction with a regular (nonfilter) needle during compounding to reduce the risk of introducing glass particulates during administration. A regular needle should be used to withdraw up to 10 mL from 1 ampule. The regular needle should be removed from the syringe and replaced with a new filter needle to inject up to 10 mL of Vitalipid N INFANT into Intralipid 20% (Fresenius Kabi). To ensure a homogeneous admixture, the bag should be inverted a couple of times immediately before the infusion. After mixing by gentle agitation, the emulsion is infused as described for Intralipid (Fresenius Kabi). Any remaining contents of opened ampules should be discarded.
- Vitalipid N INFANT has been tested in PN formulations available outside of the U.S. containing lipid injectable emulsions under European standards and demonstrated compatibility with non-U.S. approved pediatric amino acids and other PN products. Similar tests have not been conducted using U.S. amino acid products, thus caution should be taken when adding Vitalipid N INFANT to pediatric PN formulations. Branded U.S. PN products that have been tested and demonstrate compatibility include SMOFlipid, Intralipid (Fresenius Kabi).
- Results from stability studies show that unopened ampules of Vitalipid N ADULT and Vitalipid N INFANT can be stored both at +5±3°C and at +25°C/60 % RH for 24 months. Based on available data, the product is proposed to be stored between 2°C – 25°C with a shelf life of 24 months. Do not freeze. Protect the product from light
- Storage with dilution and admixture: The admixing of Vitalipid N ADULT and Vitalipid N INFANT in PN formulations has been investigated according to standard methods, documenting the physicochemical stability of admixtures after storage times up to 7 days. Vitalipid N ADULT and Vitalipid N INFANT have been tested with a variety of PN products marketed in the US, including Kabiven, PeriKabiven, and Intralipid 20% (Fresenius Kabi). The storage time and conditions specifically investigated compatibility of these products for 7 days, i.e., 6 days storage at 2°C -8°C followed by 24 hours at 20°C-25°C (i.e., 6+1 conditions). The methods employed are measurement of mean droplet size, droplet size distribution, pH, visual inspection and investigations aiming to determine precipitation in the aqueous phase. The latter have been carried out by comparisons with the corresponding aqueous systems without added lipid emulsion. The experimental results demonstrate that all tested compositions are stable and compatible under 6+1 conditions. It is important to note that there is variability in the U.S. use of different PN components which may not have been tested. If HCPs have specific questions based on their PN admixtures, the Fresenius Kabi Medical Information department can provide them with the information on the specific admixtures tested.
- Consult Fresenius Kabi for further information on complete and balanced intravenous nutrition regimens.
Product Comparison Table
Product Name U.S. FDA-Approved Product (Infuvite Adult)1 Imported Product (Vitalipid N Adult)2,3 U.S. FDA-Approved Product (Infuvite Pediatric)4 Imported Product (Vitalipid N Infant)5,6 Manufacturer Baxter Fresenius Kabi Baxter Fresenius Kabi Multivitamins & active ingredient salt forms present 13 vitamins present (water and fat soluble)
These include:
Thiamine (Vitamin B1)
Riboflavin (Vitamin B2)
Niacinamide (Vitamin B3)
Dexpanthenol (Vitamin B5)
Pyridoxine HCl (Vitamin B6)
Biotin
Folate
Cyanocobalamin (Vitamin B12)
Ascorbic acid (Vitamin C)
Vitamin A (as palmitate)
cholecalciferol (Vitamin D3)
dl-Alpha-tocopherol acetate (Vitamin E)
Vitamin K1Only fat-soluble vitamins
These include:
Retinol palmitate (vitamin A)
Ergocalciferol (Vitamin D2)
All-rac-Alpha-tocopherol (Vitamin E)
Phytomenadione (Vitamin K1)13 vitamins present (water and fat soluble)
These include:
Thiamine (vitamin B1)
Riboflavin (vitamin B2)
Niacinamide (vitamin B3)
Dexpanthenol (vitamin B5)
Pyridoxine (vitamin B6)
Biotin (B7)
Folate (B9)
Cyanocobalamin (B12)
Ascorbic acid (Vitamin C)
Vitamin A (as palmitate)
cholecalciferol (Vitamin D3)
dl-Alpha-tocopherol acetate (Vitamin E)
Vitamin K1Only fat-soluble vitamins
These include:
Retinol palmitate (vitamin A)
Ergocalciferol (Vitamin D2)
All-rac-Alpha-tocopherol (Vitamin E)
Phytomenadione (Vitamin K1)Differences in the amount of vitamins present in each 1 mL Ascorbic acid: 20 mg
Vitamin A: 330 IU
Vitamin D3: 20 IU
Thiamine (vitamin B1): 0.6 mg
Riboflavin (Vitamin B2): 0.36 mg
Pyridoxine (vitamin B6): 0.6 mg
Niacinamide: 4 mg
Dexpanthenol: 1.5 mg
Vitamin E: 1 IU
Vitamin K1: 15 mcg
Folic Acid: 60 mcg
Biotin: 6 mcg
Vitamin B12: 0.5 mcgAscorbic acid: 0
Vitamin A: 330 IU
Vitamin D2: 20 IU
Thiamine: 0
Riboflavin:0
Pyridoxine: 0
Niacinamide: 0
Dexpanthenol: 0
Vitamin E: 1 IU
Vitamin K1: 15 mcg
Folic Acid: 0
Biotin: 0
Vitamin B12: 0Ascorbic acid: 16 mg
Vitamin A: 460 IU
Vitamin D3: 80 IU
Thiamine: 0.24 mg
Riboflavin: 0.28 mg
Pyridoxine: 0.2 mg
Niacinamide: 3.4 mg
Dexpanthenol: 1 mg
Vitamin E: 1.4 IU
Vitamin K1: 40 mcg
Folic Acid: 28 mcg
Biotin: 4 mcg
Vitamin B12: 0.2 mcgAscorbic acid: 0
Vitamin A: 230 IU
Vitamin D2: 40 IU
Thiamine: 0
Riboflavin: 0
Pyridoxine: 0
Niacinamide: 0
Dexpanthenol: 0
Vitamin E: 0.7 IU
Vitamin K1: 20 mcg
Folic Acid: 0
Biotin: 0
Vitamin B12: 0Package & Container Size Single dose Vial 1: 5 mL, Vial 2: 5 mL
Pharmacy Bulk: Vial 1 – 50 mL,
Vial 2 – 50 mL10 mL glass ampule, concentrated emulsion for injection Single dose: Vial 1: 5 mL, Vial 2: 5 mL
Pharmacy Bulk: Vial 1 (40 mL Fill in 50 mL Vial) and Vial 2 (10 mL).
10 mL glass ampule, concentrated emulsion for injection Storage Minimize exposure of INFUVITE ADULT to light because vitamins A, D and riboflavin are light sensitive.
Store under refrigeration, 2-8°C (36-46°F).Between 2-25°C (36-77°F). Protect from light. Do not freeze Minimize exposure of INFUVITE PEDIATRIC to light because vitamins A, D and riboflavin are light sensitive. Store under refrigeration 2-8°C (36-46°F). Between 2- 25°C (36-77°F). Protect from light. Do not freeze Shelf Life Not reported 24 months Not reported 24 months Excipients Vial 1: polysorbate 80, sodium hydroxide and/or hydrochloric acid for pH adjustment, water for injection
Vial 2: Propylene glycol, citric acid ± sodium citrate for pH, water for injectionSoya oil
Egg lecithin
Glycerol
SWFI
NaOH for pHpolysorbate 80, sodium hydroxide and/or hydrochloric acid for pH adjustment, and water for injection.
Vial 2: mannitol, citric acid and/or sodium citrate for pH adjustment and water for injection.Soya oil
Egg lecithin
Glycerol
SWFI
NaOH for pHAluminum ≤ 70 mcg/L (vials 1+2) Not reported ≤ 30 mcg/L (Vials 1 + 2). Not reported Indication For prevention of vitamin deficiency in adults and
children aged 11 and older receiving parenteral nutritionIndicated in adult patients and children from 11 years of age as a supplement in intravenous nutrition to meet the daily requirements of the fat-soluble vitamins A, D2, E and K1. For the prevention of vitamin deficiency in
pediatric patients up to 11 years of age receiving parenteral nutritionIndicated in infants and children up to 11 years of age as a supplement in intravenous nutrition to meet the daily requirements of the fat-soluble vitamins A, D2, E and K1. Dose 10 mL 10 mL <1 kg: 1.5 mL
1 to <3 kg: 3.25 mL
≥3 kg: 5 mLPreterm and infants below 2.5 kg: 4 mL/kg/d
Infants and children >2.5 kg and up to 11 years: 10 mL/dContraindications An existing hypervitaminosis, or
A history of hypersensitivity due to any vitamins or excipients contained in this
formulation.Egg, soy, peanut allergy, hypersensitivity to ingredient/excipient An existing hypervitaminosis, or A history of hypersensitivity to any vitamins or excipients contained in this
formulation.Egg, soy, peanut allergy, hypersensitivity to ingredient/excipient The following 2 tables compare the content of Infuvite, Vitalipid N and ASPEN Vitamin Recommendations for adult and pediatric patients.
Daily Adult ASPEN Guideline Recommendations for Multivitamins and amount in Vitalipid N Adult and Infuvite Adult Products Adult: Guideline Recommendations7 Vitalipid N Adult2,3
(10 mL dose)Infuvite Adult1
(10 mL dose)Thiamine (B1) 6 mg 6 mg Riboflavin (B2) 3.6 mg 3.6 mg Niacin (B3) 40 mg 40 mg Pantothenic Acid (B5) 5 mg 15 mg Pyridoxine (B6) 6 mg 6 mg Biotin 60 mcg 60 mcg Folate 600 mcg 600 mcg Cyanocobalamin (B12) 5 mcg 5 mcg Vitamin C (ascorbic acid) 200 mg 200 mg Vitamin A 990 mcg (3300 IU) 990 mcg (3300 IU) 3300 IU (1 mg) Vitamin D 5 mcg (200 IU) 5 mcg (200 IU) 200 IU (5 mcg) Vitamin E 10 mg (10 IU) 9.1 mg (10 IU) 10 IU (10 mg) Vitamin K 150 mcg 150 mcg 150 mcg Daily Pediatric ASPEN Guideline Recommendations for Parenteral Multivitamins and amount in Vitalipid N Infant and Infuvite Pediatric Products Preterm Neonates: Guideline Recommendations Infants: Guideline Recommendations7 Children: Guideline Recommendations7 Vitalipid N Infant5,6
(10 mL)Infuvite Pediatric (4 mL+ 1 mL)4 Pediatric Dosing
Amounts/kg/d
Amounts/kg/d
Amounts/dPreterm and <2.5 kg: 4 mL/kg
>2.5 kg to 11 years: 10 mL< 1 kg: 1.5 mL
1 kg - <3 kg: 3.25 mL
≥ 3 kg: 5 mLThiamine (B1), mg 200–350 mcg 0.35 -0.5 1.2 1.2 mg Riboflavin (B2, mg) 50–200 mcg 0.15 – 0.2 1.4 1.4 mg Niacin (B3), mg 4–6.8 4 – 6.8 17 17 mg Pantothenic Acid (B5), mg 1–2 1 – 2 5 5 mg Pyridoxine (B6), mg 150–200 mcg 0.15 – 0.2 1 1 mg Biotin , mg 5-8 mcg 5 – 8 20 20 mcg Folate, mcg 56 mcg 56 140 140 mcg Cyanocobalamin (B12), mcg 0.3 0.3 1 1 mcg Vitamin C (ascorbic acid), mg 15-25 15 – 25 80 80 mg Vitamin A , mcg (IU) 700-1500 IU 150-300 (500 – 1000 IU) 150 (500 IU) 690 (2300 IU) 700 mcg (2300 IU) Vitamin D, mcg (IU) 40-160 IU 0.8 (32 IU) 10 (400 IU) 10 mcg (400 IU) 10 mcg (400 IU) Vitamin E , mg 2.8-3.5 IU 2.8 – 3.5 7 6.4 mg (7 IU) 7 mg (7 IU) Vitamin K1 , mcg 10 10 200 200 mcg 200 mcg Refer to the Vitalipid N package insert for full prescribing information REPORTING ADVERSE EVENTS
To report adverse events experienced with the use of this product, call Fresenius Kabi USA Vigilance at 1-800-551-7176, Monday – Friday, between the hours of 8 a.m. and 5 p.m. (CST), or e-mail adverse.events.USA@fresenius-kabi.com
To report a product complaint with the use of this product, call (800) 551- 7176 or e-mail productcomplaint.USA@fresenius-kabi.com.
Fresenius Kabi USA CONTACT NUMBERS: Please use the following contact numbers as appropriate:
Reason To Call Department Number ADE Reporting Vigilance Department 1-800-551-7176 Clinical/Technical Info. Or Product Complaint Medical Affairs Department Product Availability & Ordering Customer Service Department 1-888-386-1300 Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/medwatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178 (1-800-332-0178).
Sincerely,
Gordon S. Sacks, PharmD, BCNSP, FASPEN, FCCP
Senior Director, Medical Affairs
Fresenius Kabi USA, LLCReferences:
- Infuvite Adult. Prescribing Information. Baxter Healthcare Corporation. Deerfield IL. October 2016.
- Vitalipid N Adult Summary of Product Characteristics. Fresenius Kabi 2008
- Vitalipid N Adult Prescribing Information, Fresenius Kabi AB. Uppsala, Sweden
- Infuvite Pediatric. Prescribing Information. Baxter Healthcare Corporation. Deerfield IL. October 2016.
- Vitalipid N Infant Prescribing Information, Fresenius Kabi AB. Uppsala, Sweden
- Vitalipid N Infant Summary of Product Characteristics. Fresenius Kabi 2008
- Vanek VW, Borum P, Buchman A, et al. A.S.P.E.N. Position Paper: Recommendations for changes in commercially available parenteral multivitamin and multi-trace element products. Nutr Clin Pract. 2012;27(4):440-491.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VITALIPID N
vitamin a palmitate, ergocalciferol, .alpha.-tocopherol, dl-, phytonadione emulsionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65219-337 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 230 [iU] in 1 mL Ergocalciferol (UNII: VS041H42XC) (Ergocalciferol - UNII:VS041H42XC) Ergocalciferol 40 [iU] in 1 mL .ALPHA.-TOCOPHEROL, DL- (UNII: 7QWA1RIO01) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 0.7 [iU] in 1 mL PHYTONADIONE (UNII: A034SE7857) (PHYTONADIONE - UNII:A034SE7857) PHYTONADIONE 20 ug in 1 mL Inactive Ingredients Ingredient Name Strength EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) GLYCERIN (UNII: PDC6A3C0OX) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) SOYBEAN OIL (UNII: 241ATL177A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65219-337-62 10 in 1 CARTON 08/18/2023 1 NDC:65219-337-10 10 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 08/18/2023 VITALIPID N
vitamin a palmitate, ergocalciferol, .alpha.-tocopherol, dl-, phytonadione emulsionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65219-339 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 230 [iU] in 1 mL Ergocalciferol (UNII: VS041H42XC) (Ergocalciferol - UNII:VS041H42XC) Ergocalciferol 40 [iU] in 1 mL .ALPHA.-TOCOPHEROL, DL- (UNII: 7QWA1RIO01) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 0.7 [iU] in 1 mL PHYTONADIONE (UNII: A034SE7857) (PHYTONADIONE - UNII:A034SE7857) PHYTONADIONE 20 ug in 1 mL Inactive Ingredients Ingredient Name Strength EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) GLYCERIN (UNII: PDC6A3C0OX) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) SOYBEAN OIL (UNII: 241ATL177A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65219-339-64 10 in 1 CARTON 08/18/2023 1 NDC:65219-339-10 10 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 08/18/2023 VITALIPID N
vitamin a palmitate, ergocalciferol, .alpha.-tocopherol, dl-, phytonadione emulsionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65219-335 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 330 [iU] in 1 mL Ergocalciferol (UNII: VS041H42XC) (Ergocalciferol - UNII:VS041H42XC) Ergocalciferol 20 [iU] in 1 mL .ALPHA.-TOCOPHEROL, DL- (UNII: 7QWA1RIO01) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 1 [iU] in 1 mL PHYTONADIONE (UNII: A034SE7857) (PHYTONADIONE - UNII:A034SE7857) PHYTONADIONE 15 ug in 1 mL Inactive Ingredients Ingredient Name Strength EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) GLYCERIN (UNII: PDC6A3C0OX) SOYBEAN OIL (UNII: 241ATL177A) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65219-335-82 10 in 1 CARTON 08/18/2023 1 NDC:65219-335-10 10 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 08/18/2023 Labeler - Fresenius Kabi USA, LLC (013547657) Establishment Name Address ID/FEI Business Operations Fresenius Kabi AB 559785113 ANALYSIS(65219-337, 65219-339, 65219-335) , API MANUFACTURE(65219-337, 65219-339, 65219-335) , MANUFACTURE(65219-337, 65219-339, 65219-335)