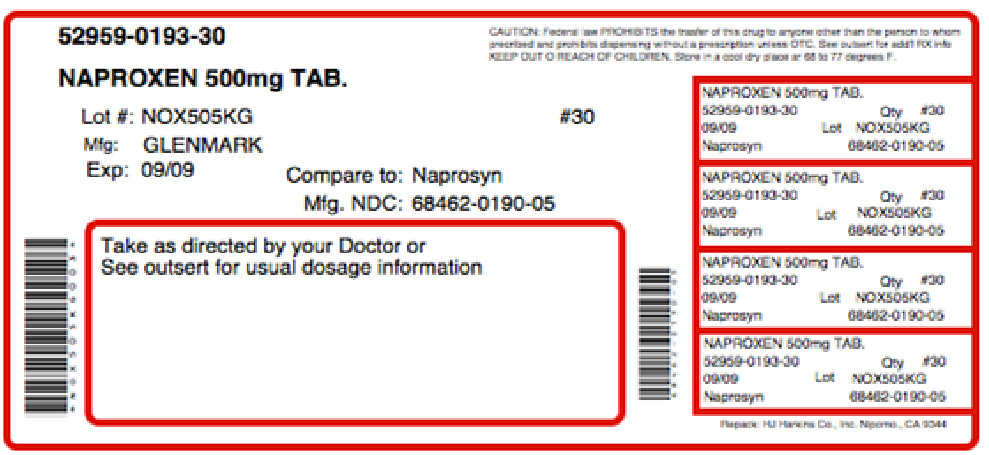
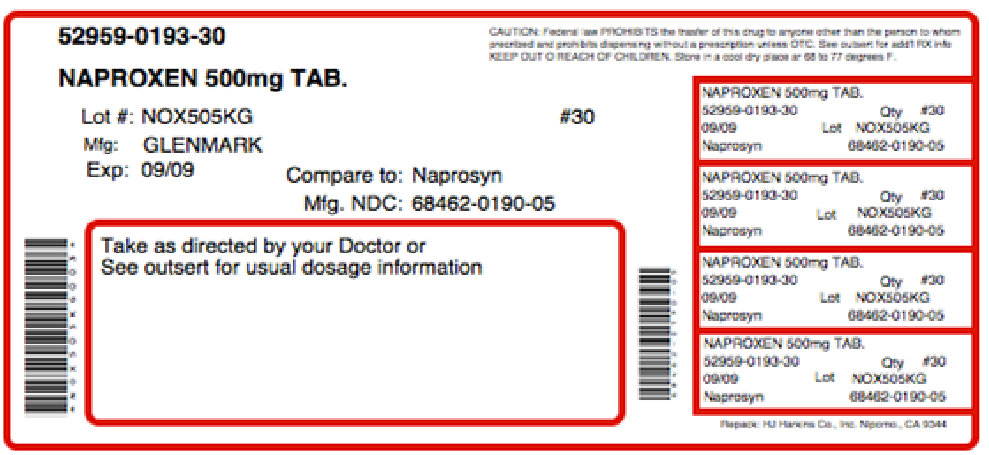
Label: THERAPROXEN-500- naproxen, .gamma.-aminobutyric acid kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 52959-193-30, 68405-118-36 - Packager: Physician Therapeutics LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 1, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
DESCRIPTION
DESCRIPTION
Naproxen USP is a proprionic acid derivative related to the arylacetic acid group of nonsteroidal anti-inflammatory drugs. The chemical names for naproxen USP and naproxen sodium USP are (S)-6-methoxy-a-methyl-2-naphthaleneacetic acid and (S)-6-methoxy-a-methyl-2-naphthaleneacetic acid, sodium salt, respectively. Naproxen USP and naproxen sodium USP have the following structures, respectively:
Naproxen USP has a molecular weight of 230.26 and a molecular formula of C14H14O3. Naproxen sodium USP has a molecular weight of 252.23 and a molecular formula of C14H13NaO3. Naproxen USP is an odorless, white to off-white crystalline substance. It is lipid-soluble, practically insoluble in water at low pH and freely soluble in water at high pH. The octanol/water partition coefficient of naproxen USP at pH 7.4 is 1.6 to 1.8. Naproxen sodium USP is a white to creamy white, crystalline solid, freely soluble in water at neutral pH. Naproxen tablets USP are available as light orange colored tablets containing 250 mg of naproxen USP, light orange colored tablets containing 375 mg of naproxen USP and light orange colored tablets containing 500 mg of naproxen USP for oral administration. The inactive ingredients are microcrystalline cellulose, croscarmellose sodium, iron oxides, povidone and magnesium stearate. Naproxen sodium tablets USP are available as blue tablets containing 275 mg of naproxen sodium USP and as blue tablets containing 550 mg of naproxen sodium USP for oral administration. The inactive ingredients are croscarmellose sodium, colloidal silicon dioxide, povidone, magnesium stearate, microcrystalline cellulose and talc. The coating suspension for the naproxen sodium 275 mg tablet may contain Opadry blue 03F50544. The coating suspension for the naproxen sodium 550 mg tablet may contain Opadry blue 03F50544.
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
Pharmacodynamics
Naproxen is a nonsteroidal anti-inflammatory drug (NSAID) with analgesic and antipyretic properties. The sodium salt of naproxen has been developed as a more rapidly absorbed formulation of naproxen for use as an analgesic. The mechanism of action of the naproxen anion, like that of other NSAIDs, is not completely understood but may be related to prostaglandin synthetase inhibition.
-
PHARMACOKINETICS
Pharmacokinetics
Naproxen and naproxen sodium are rapidly and completely absorbed from the gastrointestinal tract with an in vivo bioavailability of 95%. The different dosage forms of naproxen are bioequivalent in terms of extent of absorption (AUC) and peak concentration(Cmax); however, the products do differ in their pattern of absorption. These differences between naproxen products are related to both the chemical form of naproxen used and its formulation. Even with the observed differences in pattern of absorption, the elimination half-life of naproxen is unchanged across products ranging from 12 to 17 hours. Steady-state levels of naproxen are reached in 4 to 5 days, and the degree of naproxen accumulation is consistent with this half-life. This suggests that the differences in pattern of release play only a negligible role in the attainment of steady-state plasma levels.
Absorption
Immediate Release
After administration of naproxen tablets, peak plasma levels are attained in 2 to 4 hours. After oral administration of naproxen sodium, peak plasma levels are attained in 1 to 2 hours. The difference in rates between the two products is due to the increased aqueous solubility of the sodium salt of naproxen used in naproxen sodium.
Distribution
Naproxen has a volume of distribution of 0.16 L/kg. At therapeutic levels naproxen is greater than 99% albumin-bound. At doses of naproxen greater than 500 mg/day there is less than proportional increase in plasma levels due to an increase in clearance caused by saturation of plasma protein binding at higher doses (average trough Css 36.5, 49.2 and 56.4 mg/L with 500, 1000 and 1500 mg daily doses of naproxen, respectively). The naproxen anion has been found in the milk of lactating women at a concentration equivalent to approximately 1% of maximum naproxen concentration in plasma (see PRECAUTIONS, Nursing mothers).
Metabolism
Naproxen is extensively metabolized in the liver to 6-0-desmethyl naproxen, and both parent and metabolites do not induce metabolizing enzymes. Both naproxen and 6-0-desmethyl naproxen are further metabolized to their respective acylglucuronide conjugated metabolites.
Excretion
The clearance of naproxen is 0.13 mL/min/kg. Approximately 95% of the naproxen from any dose is excreted in the urine, primarily as naproxen (less than1%), 6-0-desmethyl naproxen (less than1%) or their conjugates (66% to 92%). The plasma half-life of the naproxen anion in humans ranges from 12 to 17 hours. The corresponding half-lives of both naproxen’s metabolites and conjugates are shorter than 12 hours, and their rates of excretion have been found to coincide closely with the rate of naproxen disappearance from the plasma. Small amounts, 3% or less of the administered dose, are excreted in the feces. In patients with renal failure metabolites may accumulate (see WARNINGS: Renal Effects).
Special Populations
Pediatric Patients
In pediatric patients aged 5 to 16 years with arthritis, plasma naproxen levels following a 5 mg/kg single dose of naproxen suspension (see DOSAGE AND ADMINISTRATION) were found to be similar to those found in normal adults following a 500 mg dose. The terminal half-life appears to be similar in pediatric and adult patients. Pharmacokinetic studies of naproxen were not performed in pediatric patients younger than 5 years of age. Pharmacokinetic parameters appear to be similar following administration of naproxen suspension or tablets in pediatric patients.
Geriatric Patients
Studies indicate that although total plasma concentration of naproxen is unchanged, the unbound plasma fraction of naproxen is increased in the elderly, although the unbound fraction is less than1% of the total naproxen concentration. Unbound trough naproxen concentrations in elderly subjects have been reported to range from 0.12% to 0.19% of total naproxen concentration, compared with 0.05% to 0.075% in younger subjects. The clinical significance of this finding is unclear, although it is possible that the increase in free naproxen concentration could be associated with an increase in the rate of adverse events per a given dosage in some elderly patients.
Race
Pharmacokinetic differences due to race have not been studied.
Hepatic Insufficiency
Naproxen pharmacokinetics has not been determined in subjects with hepatic insufficiency.
Renal Insufficiency
Naproxen pharmacokinetics has not been determined in subjects with renal insufficiency. Given that naproxen, its metabolites and conjugates are primarily excreted by the kidney, the potential exists for naproxen metabolites to accumulate in the presence of renal insufficiency. Elimination of naproxen is decreased in patients with severe renal impairment. Naproxen-containing products are not recommended for use in patients with moderate to severe and severe renal impairment (creatinine clearance less than 30 mL/min) (see WARNINGS: Renal Effects).
-
CLINICAL STUDIES
CLINICAL STUDIES
General Information
Naproxen has been studied in patients with rheumatoid arthritis, osteoarthritis, juvenile arthritis, ankylosing spondylitis, tendonitis and bursitis, and acute gout. Improvement in patients treated for rheumatoid arthritis was demonstrated by a reduction in joint swelling, a reduction in duration of morning stiffness, a reduction in disease activity as assessed by both the investigator and patient, and by increased mobility as demonstrated by a reduction in walking time. Generally, response to naproxen has not been found to be dependent on age, sex, severity or duration of rheumatoid arthritis.
In patients with osteoarthritis, the therapeutic action of naproxen has been shown by a reduction in joint pain or tenderness, an increase in range of motion in knee joints, increased mobility as demonstrated by a reduction in walking time, and improvement in capacity to perform activities of daily living impaired by the disease.
In a clinical trial comparing standard formulations of naproxen 375 mg bid (750 mg a day) vs 750 mg bid (1500 mg/day), 9 patients in the 750 mg group terminated prematurely because of adverse events. Nineteen patients in the 1500 mg group terminated prematurely because of adverse events. Most of these adverse events were gastrointestinal events.
In clinical studies in patients with rheumatoid arthritis, osteoarthritis, and juvenile arthritis, naproxen has been shown to be comparable to aspirin and indomethacin in controlling the aforementioned measures of disease activity, but the frequency and severity of the milder gastrointestinal adverse effects (nausea, dyspepsia, heartburn) and nervous system adverse effects (tinnitus, dizziness, lightheadedness) were less in naproxen-treated patients than in those treated with aspirin or indomethacin.
In patients with ankylosing spondylitis, naproxen has been shown to decrease night pain, morning stiffness and pain at rest. In doubleblind studies the drug was shown to be as effective as aspirin, but with fewer side effects.
In patients with acute gout, a favorable response to naproxen was shown by significant clearing of inflammatory changes (eg, decrease in swelling, heat) within 24 to 48 hours, as well as by relief of pain and tenderness.
Naproxen has been studied in patients with mild to moderate pain secondary to postoperative, orthopedic, postpartum episiotomy and uterine contraction pain and dysmenorrhea. Onset of pain relief can begin within 1 hour in patients taking naproxen and within 30 minutes in patients taking naproxen sodium. Analgesic effect was shown by such measures as reduction of pain intensity scores, increase in pain relief scores, decrease in numbers of patients requiring additional analgesic medication, and delay in time to remedication. The analgesic effect has been found to last for up to 12 hours.
Naproxen may be used safely in combination with gold salts and/or corticosteroids; however, in controlled clinical trials, when added to the regimen of patients receiving corticosteroids, it did not appear to cause greater improvement over that seen with corticosteroids alone. Whether naproxen has a “steroid-sparing” effect has not been adequately studied. When added to the regimen of patients receiving gold salts, naproxen did result in greater improvement. Its use in combination with salicylates is not recommended because there is evidence that aspirin increases the rate of excretion of naproxen and data are inadequate to demonstrate that naproxen and aspirin produce greater improvement over that achieved with aspirin alone. In addition, as with other NSAIDs, the combination may result in higher frequency of adverse events than demonstrated for either product alone.
In 51Cr blood loss and gastroscopy studies with normal volunteers, daily administration of 1000 mg of naproxen as 1000 mg of naproxen tablets or 1100 mg of naproxen sodium tablets has been demonstrated to cause statistically significantly less gastric bleeding and erosion than 3250 mg of aspirin.
Three 6-week, double-blind, multicenter studies with naproxen delayed release (375 or 500 mg bid, n=385) and naproxen (375 or 500 mg bid, n=279) were conducted comparing naproxen delayed release with naproxen including 355 rheumatoid arthritis and osteoarthritis patients who had a recent history of NSAID-related GI symptoms. These studies indicated that naproxen delayed release and naproxen showed no significant differences in efficacy or safety and had similar prevalence of minor GI complaints. Individual patients, however, may find one formulation preferable to the other.Geriatric Patients
The hepatic and renal tolerability of long-term naproxen administration was studied in two double-blind clinical trials involving 586 patients. Of the patients studied, 98 patients were age 65 and older and 10 of the 98 patients were age 75 and older. Naproxen was administered at doses of 375 mg twice daily or 750 mg twice daily for up to 6 months. Transient abnormalities of laboratory tests assessing hepatic and renal function were noted in some patients, although there were no differences noted in the occurrence of abnormal values among different age groups.
-
INDICATIONS & USAGE
INDICATIONS AND USAGE
Carefully consider the potential benefits and risks of naproxen, naproxen sodium and other treatment options before deciding to use naproxen and naproxen sodium tablets. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS).
Naproxen as naproxen or naproxen sodium tablets are indicated:
- For the relief of the signs and symptoms of rheumatoid arthritis
- For the relief of the signs and symptoms of osteoarthritis
- For the relief of the signs and symptoms of ankylosing spondylitis
- For the relief of the signs and symptoms of juvenile arthritis
Naproxen as naproxen suspension is recommended for juvenile rheumatoid arthritis in order to obtain the maximum dosage flexibility based on the patient’s weight.
Naproxen as naproxen and naproxen sodium tablets are also indicated:
- For relief of the signs and symptoms of tendonitis
- For relief of the signs and symptoms of bursitis
- For relief of the signs and symptoms of acute gout
- For the management of pain
- For the management of primary dysmenorrhea
-
CONTRAINDICATIONS
CONTRAINDICATIONS
Naproxen and naproxen sodium are contraindicated in patients with known hypersensitivity to naproxen and naproxen sodium.
Naproxen and naproxen sodium should not be given to patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic-like reactions to NSAIDs have been reported in such patients (see WARNINGS: Anaphylactoid Reactions and PRECAUTIONS: Preexisting Asthma).
Naproxen and naproxen sodium are contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS).
-
WARNINGS
WARNINGS
CARDIOVASCULAR EFFECTS
Cardiovascular Thrombotic Events
Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, myocardial infarction, and stroke, which can be fatal. All NSAIDS, both COX-2 selective and nonselective, may have a similar risk. Patients with known CV disease or risk factors for CV disease may be at greater risk. To minimize the potential risk for an adverse CV event in patients treated with an NSAID, the lowest effective dose should be used for the shortest duration possible. Physicians and patients should remain alert for the development of such events, even in the absence of previous CV symptoms. Patients should be informed about the signs and/or symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID does increase the risk of serious GI events (see Gastrointestinal Effect - Risk of Ulceration, Bleeding, and Perforation ).
Two large, controlled, clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10-14 days following CABG surgery found an increased incidence of myocardial infarction and stroke (see CONTRAINDICATIONS).
Hypertension
NSAIDs, including naproxen and naproxen sodium, can lead to onset of new hypertension or worsening of pre-existing hypertension, either of which may contribute to the increased incidence of CV events. Patients taking thiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs, including naproxen and naproxen sodium, should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy.
Congestive Heart Failure and Edema
Fluid retention, edema, and peripheral edema have been observed in some patients taking NSAIDs. Naproxen and naproxen sodium should be used with caution in patients with fluid retention, hypertension, or heart failure. Since each naproxen sodium tablet contains 25 mg or 50 mg of sodium (about 1 mEq per each 250 mg of naproxen), this could be considered in patients whose overall intake of sodium must be severely restricted.
Gastrointestinal Effects
Risk of Ulceration, Bleeding, and Perforation
NSAIDs, including naproxen and naproxen sodium, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal.
These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients, who develop a serious upper GI adverse event on NSAID therapy, is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3-6 months, and in about 2-4% of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk. The utility of periodic laboratory monitoring has not been demonstrated, nor has it been adequately assessed. Only 1 in 5 patients who develop a serious upper GI adverse event on NSAID therapy is symptomatic.
NSAIDs should be prescribed with extreme caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold increased risk for developing a GI bleed compared to patients with neither of these risk factors. Other factors that increase the risk for GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore, special care should be taken in treating this population. To minimize the potential risk for an adverse GI event in patients treated with an NSAID, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI adverse event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high risk patients, alternate therapies that do not involve NSAIDs should be considered.
Epidemiological studies, both of the case-control and cohort design, have demonstrated as association between use of psychotropic drugs that interfere with serotonin reuptake and the occurrence of upper gastrointestinal bleeding. In two studies, concurrent use of an NSAID or aspirin potentiated the risk of bleeding (see PRECAUTIONS - Drug Interactions). Although these studies focused on upper gastrointestinal bleeding, there is reason to believe that bleeding at other sites may be similarly potentiated.
NSAIDs should be given with care to patients with a history of inflammatory bowel disease (ulcerative colitis, Crohn’s disease) as their condition may be exacerbated.
Renal Effects
Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of a nonsteroidal anti-inflammatory drug may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, hypovolemia, heart failure, liver dysfunction, salt depletion, those taking diuretics and ACE inhibitors, and the elderly. Discontinuation of nonsteroidal anti-inflammatory drug therapy is usually followed by recovery to the pretreatment state (see WARNINGS: Advanced Renal Disease ).
Advanced Renal Disease
No information is available from controlled clinical studies regarding the use of naproxen or naproxen sodium in patients with advanced renal disease. Therefore, treatment with naproxen and naproxen sodium is not recommended in these patients with advanced renal disease. If naproxen or naproxen sodium therapy must be initiated, close monitoring of the patient's renal function is advisable.
Anaphylactoid Reactions
As with other NSAIDs, anaphylactoid reactions may occur in patients without known prior exposure to naproxen or naproxen sodium. Naproxen and naproxen sodium should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs (see CONTRAINDICATIONS and PRECAUTIONS: Preexisting Asthma). Emergency help should be sought in cases where an anaphylactoid reaction occurs. Anaphylactoid reactions, like anaphylaxis, may have a fatal outcome.
Skin Reactions
NSAIDs, including naproxen and naproxen sodium, can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Patients should be informed about the signs and symptoms of serious skin manifestations and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.
Pregnancy
In late pregnancy, as with other NSAIDs, naproxen and naproxen sodium should be avoided because it may cause premature closure of the ductus arteriosus.
-
PRECAUTIONS
PRECAUTIONS
General
Naproxen-containing products such as naproxen, naproxen sodium tablets and other naproxen products should not be used concomitantly since they all circulate in the plasma as the naproxen anion. Naproxen and naproxen sodium cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids and the patient should be observed closely for any evidence of adverse effects, including adrenal insufficiency and exacerbation of symptoms of arthritis. Patients with initial hemoglobin values of 10 g or less who are to receive long-term therapy should have hemoglobin values determined periodically. The pharmacological activity of naproxen and naproxen sodium in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, noninflammatory painful conditions. Because of adverse eye findings in animal studies with drugs of this class, it is recommended that ophthalmic studies be carried out if any change or disturbance in vision occurs.
Hepatic Effects
Borderline elevations of one or more liver tests may occur in up to 15% of patients taking NSAIDs including naproxen and naproxen sodium. Hepatic abnormalities may be the result of hypersensitivity rather than direct toxicity. These laboratory abnormalities may progress, may remain essentially unchanged, or may be transient with continued therapy. The SGPT (ALT) test is probably the most sensitive indicator of liver dysfunction. Notable elevations of ALT or AST (approximately three or more times the upper limit of normal) have been reported in approximately 1% of patients in clinical trials with NSAIDs. In addition, rare cases of severe hepatic reactions, including jaundice and fatal fulminant hepatitis, liver necrosis and hepatic failure, some of them with fatal outcomes have been reported.
A patient with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver test has occurred, should be evaluated for evidence of the development of more severe hepatic reaction while on therapy with naproxen or naproxen sodium. If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (eg, eosinophilia, rash, etc.), naproxen or naproxen sodium should be discontinued.
Chronic alcoholic liver disease and probably other diseases with decreased or abnormal plasma proteins (albumin) reduce the total plasma concentration of naproxen, but the plasma concentration of unbound naproxen is increased. Caution is advised when high doses are required and some adjustment of dosage may be required in these patients. It is prudent to use the lowest effective dose.
Hematological Effects
Anemia is sometimes seen in patients receiving NSAIDs, including naproxen and naproxen sodium. This may be due to fluid retention, occult or gross GI blood loss, or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs, including naproxen and naproxen sodium, should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia. NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible. Patients receiving either naproxen or naproxen sodium who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants, should be carefully monitored.
Preexisting Asthma
Patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm, which can be fatal. Since cross reactivity, including bronchospasm, between aspirin and other nonsteroidal anti-inflammatory drugs has been reported in such aspirin-sensitive patients, naproxen and naproxen sodium should not be administered to patients with this form of aspirin sensitivity and should be used with caution in patients with preexisting asthma.
-
INFORMATION FOR PATIENTS
Information for patients
Patients should be informed of the following information before initiating therapy with an NSAID and periodically during the course of ongoing therapy. Patients should also be encouraged to read the NSAID Medication Guide that accompanies each prescription dispensed.
1. Naproxen and naproxen sodium, like other NSAIDs, may cause serious CV side effects, such as MI or stroke, which may result in hospitalization and even death. Although serious CV events can occur without warning symptoms, patients should be alert for the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and should ask for medical advice when observing any indicative sign or symptoms. Patients should be apprised of the importance of this follow-up (see WARNINGS: Cardiovascular effects).
2. Naproxen and naproxen sodium, like other NSAIDs, can cause GI discomfort and, rarely, serious GI side effects, such as ulcers and bleeding, which may result in hospitalization and even death. Although serious GI tract ulcerations and bleeding can occur without warning symptoms, patients should be alert for the signs and symptoms of ulcerations and bleeding, and should ask for medical advice when observing any indicative sign or symptoms including epigastric pain, dyspepsia, melena, and hematemesis. Patients should be apprised of the importance of this follow-up (see WARNINGS: Gastrointestinal Effects: Risk of Ulceration, Bleeding, and Perforation )
3. Naproxen and naproxen sodium, like other NSAIDs, can cause serious skin side effects such as exfoliative dermatitis, SJS, and TEN, which may result in hospitalizations and even death. Although serious skin reactions may occur without warning, patients should be alert for the signs and symptoms of skin rash and blisters, fever, or other signs of hypersensitivity such as itching, and should ask for medical advice when observing any indicative signs or symptoms. Patients should be advised to stop the drug immediately if they develop any type of rash and contact their physicians as soon as possible.
4. Patients should promptly report signs or symptoms of unexplained weight gain or edema to their physicians.
5. Patients should be informed of the warning signs and symptoms of hepatotoxicity (eg nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness, and “flu-like” symptoms). If these occur, patients should be instructed to stop therapy and seek immediate medical therapy.
6. Patients should be informed of the signs of an anaphylactoid reaction (eg, difficulty breathing, swelling of the face or throat). If these occur, patients should be instructed to seek immediate emergency help (see WARNINGS).
7. In late pregnancy, as with other NSAIDs, naproxen and naproxen sodium should be avoided because it may cause premature closure of the ductus arteriosus.
8. Caution should be exercised by patients whose activities require alertness if they experience drowsiness, dizziness, vertigo or depression during therapy with naproxen.
-
LABORATORY TESTS
Laboratory tests
Because serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs or symptoms of GI bleeding. Patients on long-term treatment with NSAIDs should have their CBC and a chemistry profile checked periodically. If clinical signs and symptoms consistent with liver or renal disease develop, systemic manifestations occur (eg, eosinophilia, rash, etc.) or if abnormal liver tests persist or worsen, naproxen and naproxen sodium should be discontinued.
-
DRUG INTERACTIONS
Interactions
Drug interactions
ACE-inhibitors
Reports suggest that NSAIDs may diminish the antihypertensive effect of ACE-inhibitors. This interaction should be given consideration in patients taking NSAIDs concomitantly with ACE-inhibitors.
Antacids and Sucralfate
Concomitant administration of some antacids (magnesium oxide or aluminum hydroxide) and sucralfate can delay the absorption of naproxen.
Aspirin
When naproxen as naproxen or naproxen sodium tablet is administered with aspirin, its protein binding is reduced, although the clearance of free naproxen or naproxen sodium is not altered. The clinical significance of this interaction is not known; however, as with other NSAIDs, concomitant administration of naproxen and naproxen sodium and aspirin is not generally recommended because of the potential of increased adverse effects.
Cholestyramine
As with other NSAIDs, concomitant administration of cholestyramine can delay the absorption of naproxen.
Diuretics
Clinical studies, as well as postmarketing observations, have shown that naproxen and naproxen sodium can reduce the natriuretic effect of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with NSAIDs, the patient should be observed closely for signs of renal failure (see WARNINGS: Renal Effects ), as well as to assure diuretic efficacy.
Lithium
NSAIDs have produced an elevation of plasma lithium levels and a reduction in renal lithium clearance. The mean minimum lithium concentration increased 15% and the renal clearance was decreased by approximately 20%. These effects have been attributed to inhibition of renal prostaglandin synthesis by the NSAID. Thus, when NSAIDs and lithium are administered concurrently, subjects should be observed carefully for signs of lithium toxicity.
Methotrexate
NSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. Naproxen, naproxen sodium and other nonsteroidal anti-inflammatory drugs have been reported to reduce the tubular secretion of methotrexate in an animal model. This may indicate that they could enhance the toxicity of methotrexate. Caution should be used when NSAIDs are administered concomitantly with methotrexate.
Warfarin
The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that users of both drugs together have a risk of serious GI bleeding higher than users of either drug alone. No significant interactions have been observed in clinical studies with naproxen and coumarin-type anticoagulants. However, caution is advised since interactions have been seen with other nonsteroidal agents of this class. The free fraction of warfarin may increase substantially in some subjects and naproxen interferes with platelet function.
Selective Serotonin Reuptake Inhibitors (SSRIs)
There is an increased risk of gastrointestinal bleeding when selective serotonin reuptake inhibitors (SSRIs) are combined with NSAIDs. Caution should be used when NSAIDs are administered concomitantly with SSRIs.
Other Information Concerning Drug Interactions
Naproxen is highly bound to plasma albumin; it thus has a theoretical potential for interaction with other albumin-bound drugs such as coumarin-type anticoagulants, sulphonylureas, hydantoins, other NSAIDs, and aspirin. Patients simultaneously receiving naproxen and a hydantoin, sulphonamide or sulphonylurea should be observed for adjustment of dose if required.
Naproxen and other nonsteroidal anti-inflammatory drugs can reduce the antihypertensive effect of propranolol and other beta-blockers.
Probenecid given concurrently increases naproxen anion plasma levels and extends its plasma half-life significantly.
Drug/laboratory test interactions
Naproxen may decrease platelet aggregation and prolong bleeding time. This effect should be kept in mind when bleeding times are determined.
The administration of naproxen may result in increased urinary values for 17-ketogenic steroids because of an interaction between the drug and/or its metabolites with m-di-nitrobenzene used in this assay. Although 17-hydroxycorticosteroid measurements (Porter-Silber test) do not appear to be artifactually altered, it is suggested that therapy with naproxen be temporarily discontinued 72 hours before adrenal function tests are performed if the Porter-Silber test is to be used.
Naproxen may interfere with some urinary assays of 5-hydroxy indoleacetic acid (5HIAA).
-
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis, mutagenesis, impairment of fertility
A 2-year study was performed in rats to evaluate the carcinogenic potential of naproxen at rat doses of 8, 16, and 24 mg/kg/day (50, 100, and 150 mg/m2). The maximum dose used was 0.28 times the systemic exposure to humans at the recommended dose. No evidence of tumorigenicity was found.
-
PREGNANCY
Pregnancy
Teratogenic effects
Pregnancy Category C: Reproduction studies have been performed in rats at 20 mg/kg/day (125 mg/m2/day, 0.23 times the human systemic exposure), rabbits at 20 mg/kg/day (220 mg/m2/day, 0.27 times the human systemic exposure), and mice at 170 mg/kg/day (510 mg/m2/day, 0.28 times the human systemic exposure) with no evidence of impaired fertility or harm to the fetus due to the drug. However, animal reproduction studies are not always predictive of human response. There are no adequate and well-controlled studies in pregnant women. Naproxen and naproxen sodium should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic effects
There is some evidence to suggest that when inhibitors of prostaglandin synthesis are used to delay preterm labor there is an increased risk of neonatal complications such as necrotizing enterocolitis, patent ductus arteriosus and intracranial hemorrhage. Naproxen treatment given in late pregnancy to delay parturition has been associated with persistent pulmonary hypertension, renal dysfunction and abnormal prostaglandin E levels in preterm infants. Because of the known effects of nonsteroidal anti-inflammatory drugs on the fetal cardiovascular system (closure of ductus arteriosus), use during pregnancy (particularly late pregnancy) should be avoided.
-
LABOR & DELIVERY
Labor and delivery
In rat studies with NSAIDs, as with other drugs known to inhibit prostaglandin synthesis, an increased incidence of dystocia, delayed parturition, and decreased pup survival occurred. Naproxen-containing products are not recommended in labor and delivery because, through its prostaglandin synthesis inhibitory effect, naproxen may adversely affect fetal circulation and inhibit uterine contractions, thus increasing the risk of uterine hemorrhage. The effects of naproxen and naproxen sodium on labor and delivery in pregnant women are unknown.
-
NURSING MOTHERS
Nursing mothers
The naproxen anion has been found in the milk of lactating women at a concentration equivalent to approximately 1% of maximum naproxen concentration in plasma. Because of the possible adverse effects of prostaglandin-inhibiting drugs on neonates, use in nursing mothers should be avoided.
-
PEDIATRIC USE
Pediatric use
Safety and effectiveness in pediatric patients below the age of 2 years have not been established. Pediatric dosing recommendations for juvenile arthritis are based on well-controlled studies (see DOSAGE AND ADMINISTRATION). There are no adequate effectiveness or dose-response data for other pediatric conditions, but the experience in juvenile arthritis and other use experience have established that single doses of 2.5 to 5 mg/kg (as naproxen suspension, see DOSAGE AND ADMINISTRATION), with total daily dose not exceeding 15 mg/kg/day, are well tolerated in pediatric patients over 2 years of age.
-
GERIATRIC USE
Geriatric use
Studies indicate that although total plasma concentration of naproxen is unchanged, the unbound plasma fraction of naproxen is increased in the elderly. Caution is advised when high doses are required and some adjustment of dosage may be required in elderly patients. As with other drugs used in the elderly, it is prudent to use the lowest effective dose.
Experience indicates that geriatric patients may be particularly sensitive to certain adverse effects of nonsteroidal anti-inflammatory drugs. Elderly or debilitated patients seem to tolerate peptic ulceration or bleeding less well when these events do occur. Most spontaneous reports of fatal GI events are in the geriatric population (see WARNINGS).
Naproxen is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. Geriatric patients may be at a greater risk for the development of a form of renal toxicity precipitated by reduced prostaglandin formation during administration of nonsteroidal anti-inflammatory drugs (see WARNINGS: Renal Effects).
-
ADVERSE REACTIONS
ADVERSE REACTIONS
Adverse reactions reported in controlled clinical trials in 960 patients treated for rheumatoid arthritis or osteoarthritis are listed below.
In general, reactions in patients treated chronically were reported 2 to 10 times more frequently than they were in short-term studies in the 962 patients treated for mild to moderate pain or for dysmenorrhea. The most frequent complaints reported related to the gastrointestinal tract.
A clinical study found gastrointestinal reactions to be more frequent and more severe in rheumatoid arthritis patients taking daily doses of 1500 mg naproxen compared to those taking 750 mg naproxen (see CLINICAL PHARMACOLOGY).
In controlled clinical trials with about 80 pediatric patients and in well-monitored, open-label studies with about 400 pediatric patients with juvenile arthritis treated with naproxen, the incidence of rash and prolonged bleeding times were increased, the incidence of gastrointestinal and central nervous system reactions were about the same, and the incidence of other reactions were lower in pediatric patients than in adults.
In patients taking naproxen in clinical trials, the most frequently reported adverse experiences in approximately 1% to 10% of patients are:
Gastrointestinal (GI) Experiences, including: heartburn*, abdominal pain*, nausea*, constipation*, diarrhea, dyspepsia, stomatitis
Central Nervous System: headache*, dizziness*, drowsiness*, lightheadedness, vertigo
Dermatologic: pruritus (itching)*, skin eruptions*, ecchymoses*, sweating, purpura
Special Senses: tinnitus*, visual disturbances, hearing disturbances
Cardiovascular: edema*, palpitations
General: dyspnea*, thirst
*Incidence of reported reaction between 3% and 9%. Those reactions occurring in less than 3% of the patients are unmarked.
In patients taking NSAIDs, the following adverse experiences have also been reported in approximately 1% to 10% of patients.
Gastrointestinal (GI) Experiences, including: flatulence, gross bleeding/perforation, GI ulcers (gastric/duodenal), vomiting
General: abnormal renal function, anemia, elevated liver enzymes, increased bleeding time, rashes
The following are additional adverse experiences reported in less than1% of patients taking naproxen during clinical trials and through postmarketing reports. Those adverse reactions observed through postmarketing reports are italicized.
Body as a Whole: anaphylactoid reactions, angioneurotic edema, menstrual disorders, pyrexia (chills and fever)
Cardiovascular: congestive heart failure, vasculitis, hypertension, pulmonary edema
Gastrointestinal: gastrointestinal bleeding and/or perforation, hematemesis, pancreatitis, vomiting, colitis, exacerbation of inflammatory bowel disease (ulcerative colitis, Crohn’s disease), nonpeptic gastrointestinal ulceration, ulcerative stomatitis, esophagitis, peptic ulceration
Hepatobiliary: jaundice, abnormal liver function tests, hepatitis (some cases have been fatal)
Hemic and Lymphatic: eosinophilia, leucopenia, melena, thrombocytopenia, agranulocytosis, granulocytopenia, hemolytic anemia, aplastic anemia
Metabolic and Nutritional: hyperglycemia, hypoglycemia
Nervous System: inability to concentrate, depression, dream abnormalities, insomnia, malaise, myalgia, muscle weakness, aseptic meningitis, cognitive dysfunction, convulsions
Respiratory: eosinophilic pneumonitis, asthma
Dermatologic: alopecia, urticaria, skin rashes, toxic epidermal necrolysis, erythema multiforme, erythema nodosum, fixed drug eruption, lichen planus, pustular reaction, systemic lupus erythematoses, bullous reactions, including Stevens-Johnson syndrome, photosensitive dermatitis, photosensitivity reactions, including rare cases resembling porphyria cutanea tarda (pseudoporphyria) or epidermolysis bullosa. If skin fragility, blistering or other symptoms suggestive of pseudoporphyria occur, treatment should be discontinued and the patient monitored.
Special Senses: hearing impairment, corneal opacity, papillitis, retrobulbar optic neuritis, papilledema
Urogenital: glomerular nephritis, hematuria, hyperkalemia, interstitial nephritis, nephrotic syndrome, renal disease, renal failure, renal papillary necrosis, raised serum creatinine
Reproduction (female): infertility
In patients taking NSAIDs, the following adverse experiences have also been reported in less than1% of patients.
Body as a Whole: fever, infection, sepsis, anaphylactic reactions, appetite changes, death
Cardiovascular: hypertension, tachycardia, syncope, arrhythmia, hypotension, myocardial infarction
Gastrointestinal: dry mouth, esophagitis, gastric/peptic ulcers, gastritis, glossitis, eructation
Hepatobiliary: hepatitis, liver failure
Hemic and Lymphatic: rectal bleeding, lymphadenopathy, pancytopenia
Metabolic and Nutritional: weight changes
Nervous System: anxiety, asthenia, confusion, nervousness, paresthesia, somnolence, tremors, convulsions, coma, hallucinations
Respiratory: asthma, respiratory depression, pneumonia
Dermatologic: exfoliative dermatitis
Special Senses: blurred vision, conjunctivitis
Urogenital: cystitis, dysuria, oliguria/polyuria, proteinuria
-
OVERDOSAGE
OVERDOSAGE
Symptoms and Signs
Significant naproxen overdosage may be characterized by lethargy, dizziness, drowsiness, epigastric pain, abdominal discomfort, heartburn, indigestion, nausea, transient alterations in liver function, hypoprothrombinemia, renal dysfunction, metabolic acidosis, apnea, disorientation or vomiting. Gastrointestinal bleeding can occur. Hypertension, acute renal failure, respiratory depression, and coma may occur, but are rare. Anaphylactoid reactions have been reported with therapeutic ingestion of NSAIDs, and may occur following an overdose. Because naproxen sodium may be rapidly absorbed, high and early blood levels should be anticipated. A few patients have experienced convulsions, but it is not clear whether or not these were drug-related. It is not known what dose of the drug would be life threatening. The oral LD50 of the drug is 543 mg/kg in rats, 1234 mg/kg in mice, 4110 mg/kg in hamsters, and greater than 1000 mg/kg in dogs.Treatment
Patients should be managed by symptomatic and supportive care following a NSAID overdose. There are no specific antidotes. Hemodialysis does not decrease the plasma concentration of naproxen because of the high degree of its protein binding. Emesis and/or activated charcoal (60 to 100 g in adults, 1 to 2 g/kg in children) and/or osmotic cathartic may be indicated in patients seen within 4 hours of ingestion with symptoms or following a large overdose. Forced diuresis, alkalinization of urine or hemoperfusion may not be useful due to high protein binding.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION
Carefully consider the potential benefits and risks of naproxen, naproxen sodium and other treatment options before deciding to use naproxen and naproxen sodium tablets. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS).
After observing the response to initial therapy with naproxen or naproxen sodium the dose and frequency should be adjusted to suit an individual patient's needs. Different dose strengths and formulations (ie, tablets, suspension) of the drug are not necessarily bioequivalent. This difference should be taken into consideration when changing formulation. Although naproxen and naproxen sodium circulate in the plasma as naproxen, they have pharmacokinetic differences that may affect onset of action. Onset of pain relief can begin within 30 minutes in patients taking naproxen sodium and within 1 hour in patients taking naproxen.
The recommended strategy for initiating therapy is to choose a formulation and a starting dose likely to be effective for the patient and then adjust the dosage based on observation of benefit and/or adverse events. A lower dose should be considered in patients with renal or hepatic impairment or in elderly patients (see WARNINGS and PRECAUTIONS).
Geriatric Patients
Studies indicate that although total plasma concentration of naproxen is unchanged, the unbound plasma fraction of naproxen is increased in the elderly. Caution is advised when high doses are required and some adjustment of dosage may be required in elderly patients. As with other drugs used in the elderly, it is prudent to use the lowest effective dose. Patients With Moderate to Severe Renal Impairment Naproxen-containing products are not recommended for use in patients with moderate to severe and severe renal impairment (creatinine clearance less than30 mL/min) (see WARNINGS: Renal Effects). Rheumatoid Arthritis, Osteoarthritis and Ankylosing SpondylitisDuring long-term administration, the dose of naproxen may be adjusted up or down depending on the clinical response of the patient.Naproxen
250 mg
or 375 mg
or 500 mg
twice daily
twice daily
twice daily
Naproxen sodium
275 mg (naproxen 250 mg with 25 mg sodium)
550 mg (naproxen 500 mg with 50 mg sodium)
twice daily
twice daily
A lower daily dose may suffice for long-term administration. The morning and evening doses do not have to be equal in size and the administration of the drug more frequently than twice daily is not necessary.
In patients who tolerate lower doses well, the dose may be increased to naproxen 1500 mg/day for limited periods of up to 6 months when a higher level of anti-inflammatory/ analgesic activity is required. When treating such patients with naproxen 1500 mg/day, the physician should observe sufficient increased clinical benefits to offset the potential increased risk. The morning and evening doses do not have to be equal in size and administration of the drug more frequently than twice daily does not generally make a difference in response (see CLINICAL PHARMACOLOGY).
Juvenile Arthritis
The recommended total daily dose of naproxen is approximately 10 mg/kg given in 2 divided doses (ie, 5 mg/kg given twice a day).
Management of Pain, Primary Dysmenorrhea, and Acute Tendonitis and Bursitis
The recommended starting dose is 550 mg of naproxen sodium as naproxen sodium tablet followed by 550 mg every 12 hours or 275 mg every 6 to 8 hours as required. The initial total daily dose should not exceed 1375 mg of naproxen sodium. Thereafter, the total daily dose should not exceed 1100 mg of naproxen sodium. Because the sodium salt of naproxen is more rapidly absorbed, naproxen sodium tablets are recommended for the management of acute painful conditions when prompt onset of pain relief is desired. Naproxen may also be used for initial treatment of acute pain (see CLINICAL PHARMACOLOGY, INDICATIONS AND USAGE).
Acute Gout
The recommended starting dose is 750 mg of naproxen followed by 250 mg every 8 hours until the attack has subsided. Naproxen sodium may also be used at a starting dose of 825 mg followed by 275 mg every 8 hours.
-
HOW SUPPLIED
HOW SUPPLIED
Naproxen tablets USP:
250 mg: circular, light orange colored, flat, uncoated tablets, engraved with ‘G’ and ‘32’ on either side of break line on one side and ‘250’ on the other side. Packaged in light-resistant bottles of 100 and 500.
100’s (bottle): NDC 68462-188-01
500’s (bottle): NDC 68462-188-05
375 mg: oval, light orange colored, biconvex, uncoated tablets, engraved with ‘G 32” on one side and ‘375” on the other side.
Packaged in light-resistant bottles of 60, 100 and 500.
60’s (bottle): NDC 68462-189-60
100’s (bottle): NDC 68462-189-01
500’s (bottle): NDC 68462-189-05
500 mg: capsule shaped, light orange colored, uncoated tablets, having debossed with ‘G’ and ‘32’ on either side of break line on one side and ‘500’ on the other side. Packaged in light-resistant bottles of 30, 50, 60, 100 and 500.
30’s (bottle): NDC 68462-190-30
50’s (bottle): NDC 68462-190-50
60’s (bottle): NDC 68462-190-60
100’s (bottle): NDC 68462-190-01
500’s (bottle): NDC 68462-190-05
Naproxen sodium tablets USP:
275 mg: blue, oval, film-coated tablets with ‘G 0’ engraved on one side and ‘275’ engraved on the other side. Packaged in bottles of 100 and 500
100’s (bottle): NDC 68462-178-01
500’s (bottle): NDC 68462-178-05
550 mg: blue colored, modified capsule shaped, biconvex, film-coated tablets with ‘G breakline 0’ engraved on one side and breakline on the other side. Packaged in bottles of 100 and 500.
100’s (bottle): NDC 68462-179-01
500’s (bottle): NDC 68462-179-05 -
STORAGE AND HANDLING
Store at 15° to 30°C (59° to 86°F) in well-closed containers; dispense in light-resistant containers.
Manufactured by:
Glenmark Generics Ltd
Colvale-Bardez, Goa 403 513, India
Manufactured for:
G
Glenmark
Glenmark Generics Inc., USA
Mahwah, NJ 07430
Questions? 1 (888)721-7115
www.glenmarkgenerics.com
November 2008 -
MEDICATION GUIDE
MEDICATION GUIDE FOR NON-STEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS)
(See the end of this Medication Guide for a list of prescription NSAID medicines.)
What is the most important information I should know about medicines called Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
NSAID medicines may increase the chance of a heart attack or stroke that can lead to death. This chance increases:
- with longer use of NSAID medicines
- in people who have heart disease
NSAID medicines should never be used right before or after a heart surgery called a “coronary artery bypass graft (CABG).”
NSAID medicines can cause ulcers and bleeding in the stomach and intestines at any time during treatment. Ulcers and bleeding:
- can happen without warning symptoms
- may cause death
The chance of a person getting an ulcer or bleeding increases with:
- taking medicines called “corticosteroids” and “anticoagulants”
- longer use
- smoking
- drinking alcohol
- older age
- having poor health
NSAID medicines should only be used:
- exactly as prescribed
- at the lowest dose possible for your treatment
- for the shortest time needed
What are Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
NSAID medicines are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as:
- different types of arthritis- menstrual cramps and other types of short-term pain
Who should not take a Non-Steroidal Anti-Inflammatory Drug (NSAID)?
Do not take an NSAID medicine:
- if you had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAID medicine
- for pain right before or after heart bypass surgery
Tell your healthcare provider:
- about all your medical conditions.
- about all of the medicines you take. NSAIDs and some other medicines can interact with each other and cause serious side effects.
Keep a list of your medicines to show to your healthcare provider and pharmacist.
- if you are pregnant. NSAID medicines should not be used by pregnant women late in their pregnancy.
- if you are breastfeeding. Talk to your doctor.
What are the possible side effects of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?Get emergency help right away if you have any of the following symptoms:Serious side effects include:
Other side effects include:
heart attack
stomach pain
stroke
constipation
high blood pressure
diarrhea
heart failure from body swelling (fluid retention)
gas
kidney problems including kidney failure
heartburn
bleeding and ulcers in the stomach and intestine
nausea
low red blood cells (anemia)
vomiting
life-threatening skin reactions
dizziness
life-threatening allergic reactions
liver problems including liver failure
asthma attacks in people who have asthma
- shortness of breath or trouble breathing
- slurred speech
- chest pain
- swelling of the face or throat
- weakness in one part or side of your body
Stop your NSAID medicine and call your healthcare provider right away if you have any of the following symptoms:
- nausea
- there is blood in your bowel movement or it is black and sticky like tar
- more tired or weaker than usual
- itching
- your skin or eyes look yellow
- unusual weight gain
- stomach pain
- skin rash or blisters with fever
- flu-like symptoms
- swelling of the arms and legs, hands and feet
- vomit blood
These are not all the side effects with NSAID medicines. Talk to your healthcare provider or pharmacist for more information about NSAID medicines.
Other information about Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
- Aspirin is an NSAID medicine but it does not increase the chance of a heart attack. Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can also ccause ulcers in the stomach and intestines.
- Some of these NSAID medicines are sold in lower doses without a prescription (over- the-counter). Talk to your healthcare provider before using over-the- counter NSAIDs for more than 10 days.
NSAID medicines that need a prescription
This Medication Guide has been approved by the U.S. Food and Drug Administration.Generic Name
Tradename
Celecoxib
Celebrex®
Diclofenac
Cataflam®, Voltaren®, Arthrotec™ (combined with misoprostol) Diflunisal
Dolobid®
Etodolac
Lodine®, Lodine® XL
Fenoprofen
Nalfon®, Nalfon® 200
Flurbirofen
Ansaid®
Ibuprofen
Motrin®, Tab-Profen®, Vicoprofen** (combined with hydrocodone), CombunoxTM (combined with oxycodone)
Indomethacin
Indocin®, Indocin® SR, Indo-Lemmon™, Indomethagan™
Ketoprofen
Oruvail®
Ketorolac
Toradol®
Mefenamic Acid
Ponstel®
Meloxicam
Mobic®
Nabumetone
Relafen®
Naproxen
Naprosyn®, Anaprox®, Anaprox® DS, EC-Naproxyn®, Naprelan®, Naprapac ® (copackaged with lansoprazole)
Oxaprozin
Daypro®
Piroxicam
Feldene®
Sulindac
Clinoril®
Tolmetin Tolectin®, Tolectin DS®, Tolectin® 600
* Vicoprofen contains the same dose of ibuprofen as over-the-counter (OTC) NSAID, and is usually used for less than 10 days to treat
pain. The OTC NSAID label warns that long term continuous use may increase the risk of heart attack or stroke
* Vicoprofen contains the same dose of ibuprofen as over-the-counter (OTC) NSAID, and is usually used for less than 10 days to treat
pain. The OTC NSAID label warns that long term continuous use may increase the risk of heart attack or stroke
Manufactured by:
Glenmark Generics Ltd
Colvale-Bardez, Goa 403 513
India
Manufactured for:
Glenmark
Glenmark Generics Inc., USA
Mahwah, NJ 07430
Questions? 1 (888)721-7115
www.glenmarkgenerics.com
November 2008
-
PRINCIPAL DISPLAY PANEL
G
GlenmarkNDC 68462-190-01
NAPROXEN TABLETS USP
500 mg
PHARM ACIST:
Dispense with a Medication Guide
Rx only 100 Tablets
Each tablet contains 500 mg in naproxen USP.
USUAL DOSAGE: For dosage recommendations and other important prescribing information, read accompanying insert.
Dispense in light-resistant containers.
Manufactured by:
Glenmark Generics Ltd.
Colvale-Bardez, Goa 403 513,
IndiaGO/DRUGS/648
Manufactured for:
Glenmark Generics Inc., USA
Mahwah, NJ 07430STORE AT 15 Degree C - 30 Degree C (59 Degree to 86 Degree F)
Lot No:
Exp:
07/08
Questions? 1 (888)721-7115
www.glenmarkgenerics.com
-
SPL UNCLASSIFIED SECTION
Theramine™ PRODUCT INFORMATION Theramine (U.S. patent pending) capsules by oral administration. A specially formulated Medical Food product, consisting of a proprietary blend of amino acids and polyphenol ingredients in specific proportions, for the dietary management of the metabolic processes associated with pain disorders and inflammatory conditions. (PD) (IC). Must be administered under physician supervision. Medical Foods Medical Food products are often used in hospitals (e.g., for burn victims or kidney dialysis patients) and outside of a hospital setting under a physician’s care for the dietary management of diseases in patients with particular medical or metabolic needs due to their disease or condition. Congress defined "Medical Food" in the Orphan Drug Act and Amendments of 1988 as "a food which is formulated to be consumed or administered enterally [or orally] under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation." Medical Foods are complex formulated products, requiring sophisticated and exacting technology. Theramine has been developed, manufactured, and labeled in accordance with both the statutory and the FDA regulatory definition of a Medical Food. Theramine must be used while the patient is under the ongoing care of a physician. PAIN DISORDERS (PD) INFLAMMATORY CONDITIONS (IC) PD and IC as a Metabolic Deficiency Disease A critical component of the definition of a Medical Food is the requirement for a distinctive nutritional deficiency. FDA scientists have proposed a physiologic definition of a distinctive nutritional deficiency as follows: “the dietary management of patients with specific diseases requires, in some instances, the ability to meet nutritional requirements that differ substantially from the needs of healthy persons. For example, in establishing the recommended dietary allowances for general, healthy population, the Food and Nutrition Board of the Institute of Medicine National Academy of Sciences, recognized that different or distinctive physiologic requirements may exist for certain persons with "special nutritional needs arising from metabolic disorders, chronic diseases, injuries, premature birth, other medical conditions and drug therapies. Thus, the distinctive nutritional needs associated with a disease reflects the total amount needed by a healthy person to support life or maintain homeostasis, adjusted for the distinctive changes in the nutritional needs of the patient as a result of the effects of the disease process on absorption, metabolism and excretion.” It was also proposed that in patients with certain disease states who respond to nutritional therapies, a physiologic deficiency of the nutrient is assumed to exist. For example, if a patient with pain disorders responds to a tryptophan formulation by decreasing perceived pain, a deficiency of tryptophan is assumed to exist. Patients with pain disorders and inflammatory conditions are known to have nutritional deficiencies of tryptophan, choline, arginine, GABA, flavonoids, and certain antioxidants. Patients with pain disorders and inflammatory conditions frequently exhibit reduced plasma levels of tryptophan and GABA and have been shown to respond to oral administration of GABA, arginine, tryptophan, or a 5-hydoxytryptophan formulation. Research has shown that tryptophan, arginine or GABA reduced diets result in a fall of circulating tryptophan, arginine, and/or GABA. Patients with pain disorders frequently exhibit activation of the degradation pathways that increases the turnover of GABA, arginine and/or tryptophan leading to a reduced level of production of serotonin, GABA or nitric oxide for a given precursor blood level. Research has also shown that a genetic predisposition to accelerated degradation can lead to increased precursor requirements in certain patients with pain disorders and inflammatory conditions. Choline is required to fully potentiate acetylcholine synthesis by brain neurons. A deficiency of choline leads to reduced acetylcholine production by the neurons. Flavonoids potentiate the production of acetylcholine by the neurons thereby reducing delta pain. Diets deficient in flavonoid rich foods and choline result in inadequate flavonoid concentrations, impeding acetylcholine production in certain patients with pain disorders and/or inflammatory conditions. Acetylcholine in pre-synaptic ganglia is necessary for the production of serotonin and nitric oxide in post-synaptic ganglia. Provision of tryptophan, arginine, GABA, choline and flavonoids with antioxidants, in specific proportions can restore the production of beneficial serotonin, nitric oxide, and acetylcholine, thereby reducing the perception of pain and reducing inflammation. L-Histidine is known to produce brain histamine that stimulates production of ACTH.
-
DESCRIPTION
PRODUCT DESCRIPTION Primary Ingredients Theramine consists of a proprietary blend of amino acids, cocoa, caffeine, cinnamon, and flavonoids in specific proportions. These ingredients fall into the category of Generally Regarded as Safe” (GRAS) as defined by the Food and Drug Administration (FDA) (Sections 201(s) and 409 of the Federal Food, Drug, and Cosmetic Act). A GRAS substance is distinguished from a food additive on the basis of the common knowledge about the safety of the substance for its intended use. The standard for an ingredient to achieve GRAS status requires not only technical demonstration of non-toxicity and safety, but also general recognition of safety through widespread usage and agreement of that safety by experts in the field. Many ingredients have been determined by the U.S. Food and Drug Administration (FDA) to be GRAS, and are listed as such by regulation, in Volume 21 Code of Federal Regulations (CFR) Sections 182, 184, and 186. Amino Acids Amino Acids are the building blocks of protein. All amino acids are GRAS listed as they have been ingested by humans for thousands of years. The doses of the amino acids in Theramine are equivalent to those found in the usual human diet. Patients with pain disorders may require an increased amount of certain amino acids that cannot be obtained from normal diet alone. Tryptophan, for example, is an obligatory amino acid. The body cannot make tryptophan and must obtain tryptophan from the diet. Tryptophan is needed to produce serotonin. Serotonin is required to reduce pain. Patients with pain disorders and inflammatory conditions have altered serotonin metabolism. Some patients with pain disorders and inflammatory conditions have a resistance to the use of tryptophan that is similar to the mechanism found in insulin resistance. Patients with pain disorders and inflammatory conditions cannot acquire sufficient tryptophan from the diet to alter the perception of pain and the inflammatory process without ingesting a prohibitively large amount of calories, particularly calories from protein. Flavonoids Flavonoids are a group of phytochemical compounds found in all vascular plants including fruits and vegetables. They are a part of a larger class of compounds known as polyphenols. Many of the therapeutic or health benefits of colored fruits and vegetables, cocoa, red wine, and green tea are directly related to their flavonoid content. The specially formulated flavonoids found in Theramine cannot be obtained from conventional foods in the necessary proportions to elicit a therapeutic response. Other Ingredients Theramine contains the following inactive or other ingredients, as fillers, excipients, and colorings: magnesium stearate, microcrystalline cellulose, Maltodextrin NF, gelatin (as the capsule material). Physical Description Theramine is a yellow to light brown powder. Theramine contains L-Glutamine, L-Arginine, L-Histidine, and L-Serine, 5-Hydroxytryptophan as Griffonia Seed Extract, GABA, Choline Bitartrate, Cinnamon, Cocoa, Hydrolyzed Whey Protein, and Grape Seed Extract.
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY Mechanism of Action Theramine acts by restoring and maintaining the balance of the neurotransmitters; GABA, nitric oxide, serotonin, and acetylcholine that are associated with pain disorders and inflammatory conditions. Theramine stimulates the production ACTH to reduce inflammation. Metabolism The amino acids in Theramine are primarily absorbed by the stomach and small intestines. All cells metabolize the amino acids in Theramine. Circulating tryptophan, arginine and choline blood levels determine the production of serotonin, nitric oxide, and acetylcholine. Excretion Theramine is not an inhibitor of cytochrome P450 1A2, 2C9, 2C19, 2D6, or 3A4. These isoenzymes are principally responsible for 95% of all detoxification of drugs, with CYP3A4 being responsible for detoxification of roughly 50% of drugs. Amino acids do not appear to have an effect on drug metabolizing enzymes.
- INDICATIONS & USAGE
-
CLINICAL STUDIES
CLINICAL EXPERIENCE Administration of Theramine has demonstrated significant reduction in symptoms of pain and inflammation in patients with acute and chronic pain when used for the dietary management of the metabolic processes associated with pain disorders and inflammatory conditions. Administration of Theramine results in the induction and maintenance of pain relief in patients with pain disorders and inflammatory conditions.
- CONTRAINDICATIONS
-
ADVERSE REACTIONS
ADVERSE REACTIONS Oral supplementation with L-tryptophan, L-arginine or choline at high doses up to 15 grams daily is generally well tolerated. The most common adverse reactions of higher doses — from 15 to 30 grams daily — are nausea, abdominal cramps, and diarrhea. Some patients may experience these symptoms at lower doses. The total combined amount of amino acids in each Theramine capsule does not exceed 400 mg.
- DRUG INTERACTIONS
-
OVERDOSAGE
OVERDOSE There is a negligible risk of overdose with Theramine as the total dosage of amino acids in a one month supply (90 capsules) is less than 36 grams. Overdose symptoms may include diarrhea, weakness, and nausea. POST-MARKETING SURVEILLANCE Post-marketing surveillance has shown no serious adverse reactions. Reported cases of mild rash and itching may have been associated with allergies to Theramine flavonoid ingredients, including cinnamon, cocoa, and chocolate. These reactions were transient in nature and subsided within 24 hours.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION Recommended Administration For the dietary management of the metabolic processes associated with pain disorders and inflammatory conditions. Take (2) capsules one to three times daily or as directed by physician. As with most amino acid formulations Theramine should be taken without food to increase the absorption of key ingredients.
-
HOW SUPPLIED
How Supplied Theramine is supplied in purple and white, size 0 capsules in bottles of 60 or 90 capsules. Physician Supervision Theramine is a Medical Food product available by prescription only and must be used while the patient is under ongoing physician supervision. U.S. patent pending. Manufactured by Arizona Nutritional Supplements, Inc. Chandler AZ 85225 Distributed by Physician Therapeutics LLC, Los Angeles, CA 90077. www.ptlcentral.com Copyright 2003-2006, Physician Therapeutics LLC, all rights reserved NDC: 68405-1008-02 NDC: 68405-1008-03
- STORAGE AND HANDLING
-
PRINCIPAL DISPLAY PANEL
PHYSICIAN THERAPEUTICS THERAMINE Medical Food Rx only 90 Capsules Directions for use: Must be administered under medical supervision. For adults only. As a Medical Food, take one (1) or two (2) capsules every four hours or as directed by your medical practitioner. For the dietary management of Myalgia. Contains no added sugar, starch, wheat, yeast, preservatives, artificial flavor. Storage: Keep tightly closed in a cool dry place 8-320 C (45-900 F), relative humidity, below 50%. Warning: Keep this product out of the reach of children. NDC# 68405-1008-03 Ingredients: Each serving (per 2 capsules) contains: Proprietary Amino Acid Blend Griffonia Seed Extract (5-HTP), Whey Protein Hydrolysate, L-Arginini, L-Histidine (as L-Histidine HCl), L-Glutamine, L-Serine, Gamma Amino Butyric Acid, Choline Bitartrate, Cocoa (6% Theobromine) (fruit), Grape Extract (95% Polyphenols) (seed), Cinnamon (bark) Other Ingredients: Gelatin, Silicon Dioxide, Tricalcium phosphate, Vegetable Magnesium Stearate, Cellulose, FD and C Blue #1, FD and C red#3, titanium dioxide. Distributed by: Physician Therapeutics LLC, Los Angeles, CA 90077 www.ptlcentral.com Patent Pending
-
PRINCIPAL DISPLAY PANEL
PHYSICIAN THERAPEUTICS Theramine + Naproxen 500 mg A Convenience Packed Medical Food And Drug Theraproxen-500 PHYSICIAN THERAPEUTICS > Theramine 90 Capsules > Naproxen 500 mg 30 Tablets No Refills Without Physician Authorization Rx only NDC # 68405-118-36 of this co-pack As prescribed by physician. See product label and product information insert. Naproxen 500 mg Rx Drug For the Dietary Management Of Pain and inflammation. Two capsules twice daily or as directed by physician. See product label and insert. Theramine
Medical Food Manufactured and Distributed by Physician Therapeutics, A Division of Targeted Medical Pharma Inc. Los Angeles, CA 90077 www.ptlcentral.com B-NDC# 68405-8118-36
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
THERAPROXEN-500
naproxen, .gamma.-aminobutyric acid kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68405-118 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68405-118-36 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 30 Part 2 1 BOTTLE 90 Part 1 of 2 NAPROXEN
naproxen tabletProduct Information Item Code (Source) NDC:52959-193(NDC:68462-190) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAPROXEN (UNII: 57Y76R9ATQ) (NAPROXEN - UNII:57Y76R9ATQ) NAPROXEN 500 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) POVIDONE (UNII: FZ989GH94E) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color orange (Light Orange) Score 2 pieces Shape CAPSULE Size 16mm Flavor Imprint Code G;32;500 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52959-193-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078250 03/03/2011 Part 2 of 2 THERAMINE 90
.gamma.-aminobutyric acid capsuleProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength .GAMMA.-AMINOBUTYRIC ACID (UNII: 2ACZ6IPC6I) (.GAMMA.-AMINOBUTYRIC ACID - UNII:2ACZ6IPC6I) .GAMMA.-AMINOBUTYRIC ACID 100 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MALTODEXTRIN (UNII: 7CVR7L4A2D) GELATIN (UNII: 2G86QN327L) Product Characteristics Color green (GREEN) Score no score Shape CAPSULE Size 21mm Flavor Imprint Code ; Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Medical Food 03/03/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/03/2011 Labeler - Physician Therapeutics LLC (931940964) Establishment Name Address ID/FEI Business Operations Glenmark Generics Limited 677318665 manufacture Establishment Name Address ID/FEI Business Operations H.J. Harkins Company, Inc. 147681894 repack Establishment Name Address ID/FEI Business Operations Targeted Medical Pharma Inc. 126962740 manufacture