Label: DAYAVITE- coenzyme q10, alpha-lipoic acid, ascorbic acid, beta-carotene, cholecalciferol, chromium, copper, d-alpha tocopheryl acid, folic acid, magnesium, manganese, methylcobalamin, molybdenum amino acid, niacinamide, potassium, pyridoxine, riboflavin, selenium, thiamine, zinc tablet, coated
- NHRIC Code(s): 72287-663-30
- Packager: Amella Pharma, Llc
- Category: DIETARY SUPPLEMENT
Drug Label Information
Updated April 15, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HEALTH CLAIM:DAYAVITE Tablets Dietary Supplement
Dispensed by Prescription†
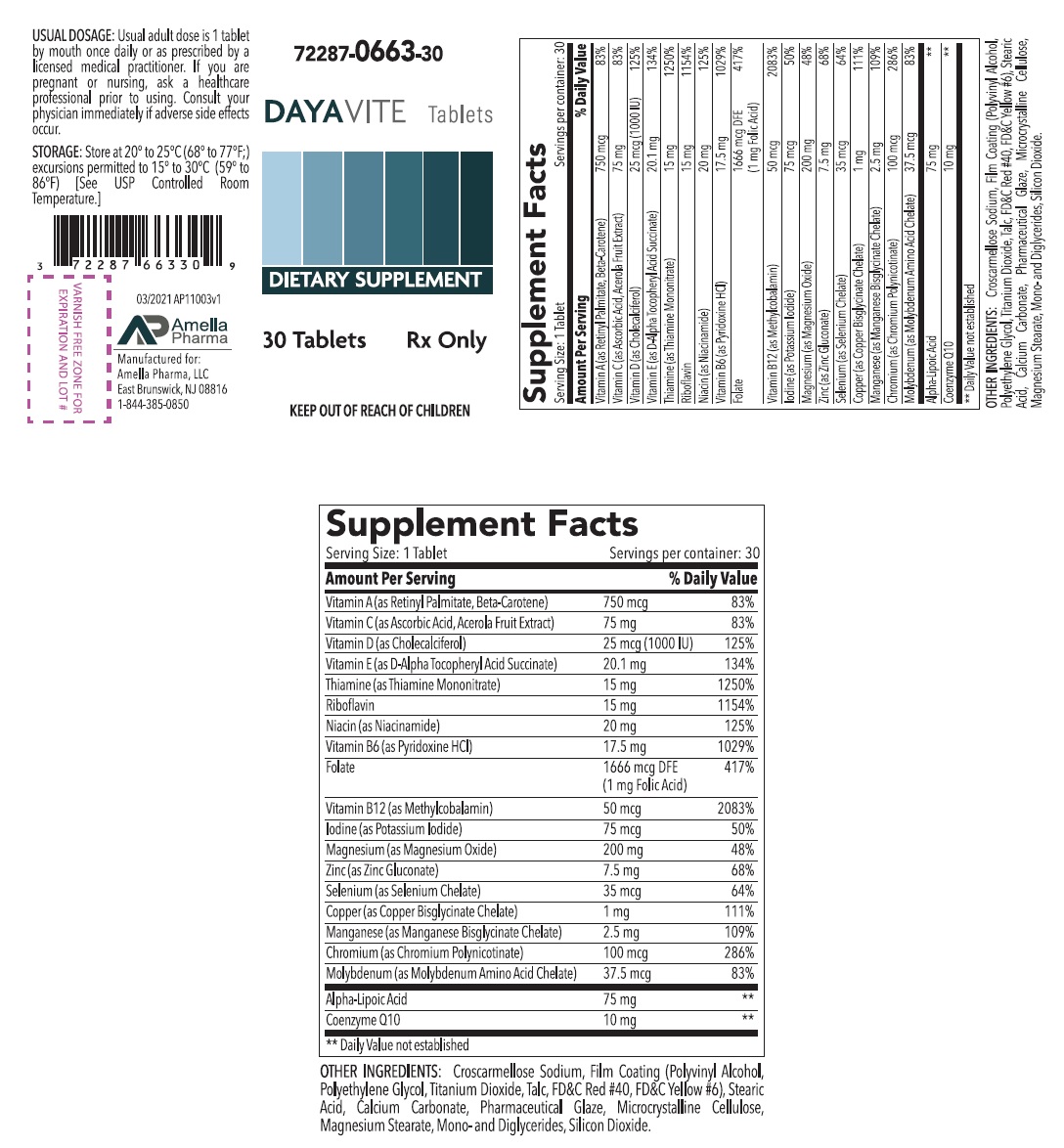
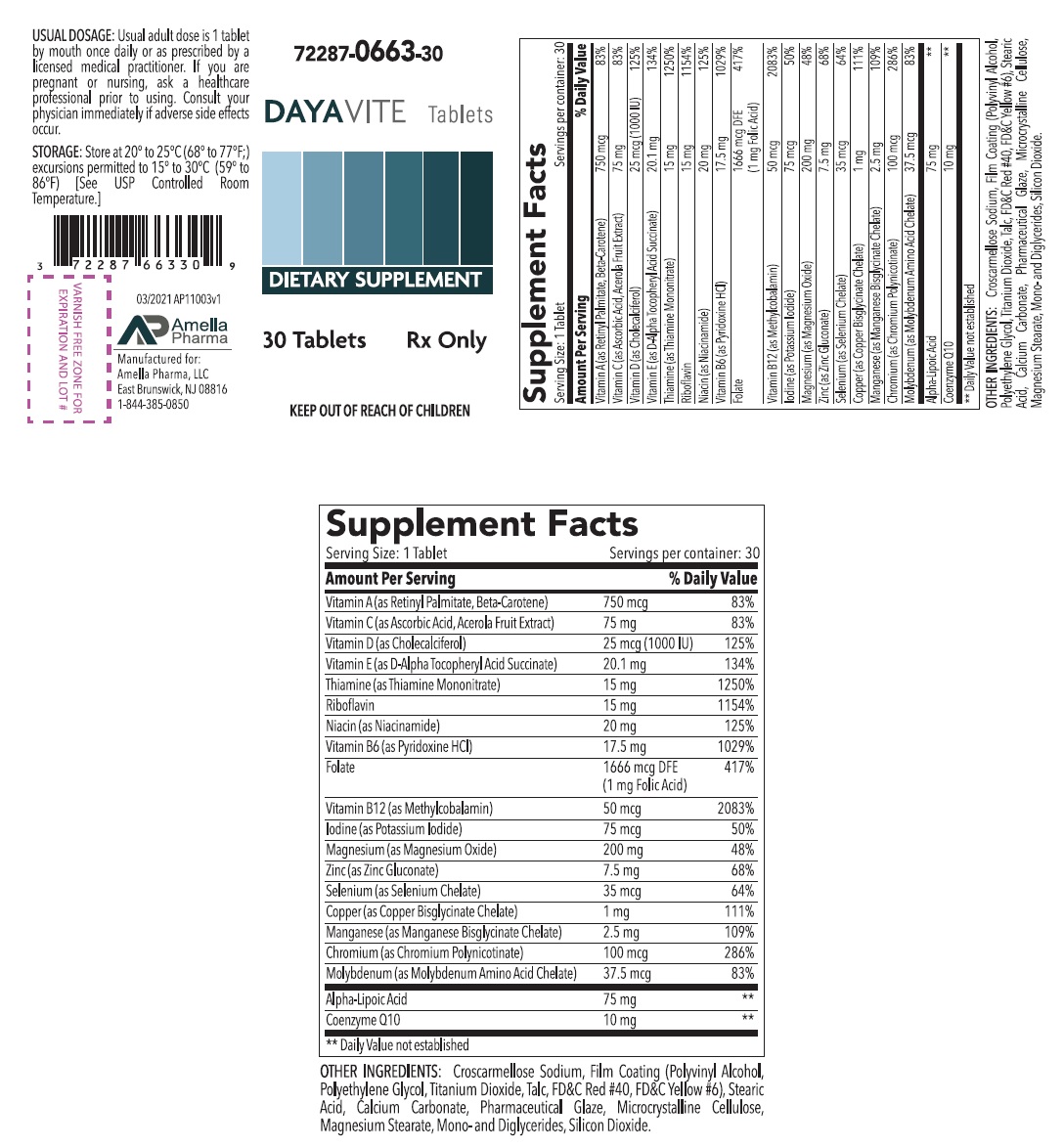
Supplement Facts Serving Size:1 Tablet Serving per container: 30 Amount Per Serving % Daily Value Vitamin A (as Retinyl Palmitate, Beta-Carotene) 750 mcg 83% Vitamin C (as Ascorbic Acid, Acerola Fruit Extract) 75 mg 83% Vitamin D (as Cholecalciferol) 25 mcg (1000 IU) 125% Vitamin E (as D-Alpha Tocopheryl Acid Succinate) 20.1 mg 134% Thiamine (as Thiamine Mononitrate) 15 mg 1250% Riboflavin 15 mg 1154% Niacin (as Niacinamide) 20 mg 125% Vitamin B6 (as Pyridoxine HCl) 17.5 mg 1029% Folate 1666 mcg DFE 417% (1 mg Folic Acid) Vitamin B12 (as Methylcobalamin) 50 mcg 2083% Iodine (as Potassium Iodide) 75 mcg 50% Magnesium (as Magnesium Oxide) 200 mg 48% Zinc (as Zinc Gluconate) 7.5 mg 68% Selenium (as Selenium Chelate) 35 mcg 64% Copper (as Copper Bisglycinate Chelate) 1 mg 111% Manganese (as Manganese Bisglycinate Chelate) 2.5 mg 109% Chromium (as Chromium Polynicotinate) 100 mcg 286% Molybdenum (as Molybdenum Amino Acid Chelate) 37.5 mcg 83% Alpha-Lipoic Acid 75 mg ** Coenzyme Q10 10 mg ** ** Daily Value not established OTHER INGREDIENTS: Croscarmellose Sodium, Film Coating (Polyvinyl Alcohol, Polyethylene Glycol, Titanium Dioxide, Talc, FD&C Red #40, FD&C Yellow #6), Stearic Acid, Calcium Carbonate, Pharmaceutical Glaze, Microcrystalline Cellulose, Magnesium Stearate, Mono-and Diglycerides, Silicon Dioxide.
-
DESCRIPTION
DAYAVITE Tablets is an orally administered prescription vitamin formulation for the clinical dietary management of suboptimal nutritional status in patients where advanced folate supplementation is required and nutritional supplementation in physiologically stressful conditions for maintenance of good health is needed.
DAYAVITE is a long, oblong, dark pink, clear-coated tablet, with debossed "A3” on one side.
-
PRECAUTIONS
CONTRAINDICATIONS
This product is contraindicated in patients with known hypersensitivity to any of the ingredients.PRECAUTIONS
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.DAYAVITE Tablets should only be used under the direction and supervision of a licensed medical practitioner. Use with caution in patients that may have a medical condition, are pregnant, lactating, trying to conceive, under the age of 18, or taking medications.
Folic acid supplementation may obscure pernicious anemia, in that hematologic remission can occur while neurological manifestations progress.
-
WARNINGS
KEEP OUT OF REACH OF CHILDREN.
Pregnancy and Lactation:
DAYAVITE Tablets is not intended for use in pregnant or lactating patients.ADVERSE REACTIONS
Allergic sensitizations have been reported following oral administration of folic acid. Consult your physician immediately if adverse side effects occur. - DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED HEALTH CLAIM:
DAYAVITE Tablets are supplied as a long, oblong, dark pink, clear-coated tablet, with debossed "A3” on one side.
DAYAVITE Tablets is available as the following:
72287-0663-30* 30ct bottleStore at 20° to 25°C (68° to 77°F; excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]
Manufactured for:
Amella Pharma, LLC
E Brunswick, NJ 08816Call your doctor for medical advice about side effects. You may report side effects to Amella Pharma, LLC at 1-844-385-0850.
Issued: 04/2021 AP11002v1
*Amella Pharma does not represent these product codes to be National Drug Codes (NDC). Product codes are formatted according to standard industry practice, to meet the formatting requirement by pedigree reporting and supply-chain control including pharmacies.
† This product is a prescription-folate with or without other dietary ingredients that – due to increased folate levels increased risk associated with masking of B12 deficiency (pernicious anemia) requires administration under the care of a licensed medical practitioner (61 FR 8760). The most appropriate way to ensure pedigree reporting consistent with these regulatory guidelines and safety monitoring is to dispense this product only by prescription (Rx). This is not an Orange Book product. This product may be administered only under a physician’s supervision and all prescriptions using this product shall be pursuant to state statutes as applicable. The ingredients, indication or claims of this product are not to be construed to be drug claims.
1. Federal Register Notice of August 2, 1973 (38 FR 20750)
2. Federal Register Notice of October 17, 1980 (45 FR 69043, 69044)
3. Federal Register Notice of March 5, 1996 (61 FR 8760) - Packaging
-
INGREDIENTS AND APPEARANCE
DAYAVITE
coenzyme q10, alpha-lipoic acid, ascorbic acid, beta-carotene, cholecalciferol, chromium, copper, d-alpha tocopheryl acid, folic acid, magnesium, manganese, methylcobalamin, molybdenum amino acid, niacinamide, potassium, pyridoxine, riboflavin, selenium, thiamine, zinc tablet, coatedProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:72287-663 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 750 ug ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 75 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 25 ug .ALPHA.-TOCOPHEROL SUCCINATE, D- (UNII: LU4B53JYVE) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL SUCCINATE, D- 20.1 mg THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 15 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 15 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 20 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 17.5 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg METHYLCOBALAMIN (UNII: BR1SN1JS2W) (METHYLCOBALAMIN - UNII:BR1SN1JS2W) METHYLCOBALAMIN 50 ug POTASSIUM IODIDE (UNII: 1C4QK22F9J) (IODIDE ION - UNII:09G4I6V86Q) IODIDE ION 75 ug MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CATION 200 mg ZINC GLUCONATE (UNII: U6WSN5SQ1Z) (ZINC CATION - UNII:13S1S8SF37) ZINC GLUCONATE 7.5 mg SELENIUM (UNII: H6241UJ22B) (SELENIUM - UNII:H6241UJ22B) SELENIUM 35 ug COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 1 mg MANGANESE (UNII: 42Z2K6ZL8P) (MANGANESE - UNII:42Z2K6ZL8P) MANGANESE 2.5 mg CHROMIUM NICOTINATE (UNII: A150AY412V) (NIACIN - UNII:2679MF687A) CHROMIUM NICOTINATE 100 ug MOLYBDENUM (UNII: 81AH48963U) (MOLYBDENUM - UNII:81AH48963U) MOLYBDENUM 37.5 ug ALPHA LIPOIC ACID (UNII: 73Y7P0K73Y) (.ALPHA.-LIPOIC ACID - UNII:73Y7P0K73Y) ALPHA LIPOIC ACID 75 mg UBIDECARENONE (UNII: EJ27X76M46) (UBIDECARENONE - UNII:EJ27X76M46) UBIDECARENONE 10 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TALC (UNII: 7SEV7J4R1U) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) STEARIC ACID (UNII: 4ELV7Z65AP) CALCIUM CARBONATE (UNII: H0G9379FGK) SHELLAC (UNII: 46N107B71O) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) GLYCERYL MONO AND DIPALMITOSTEARATE (UNII: KC98RO82HJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:72287-663-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 04/14/2021 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 19 mm scoring 1 imprint Labeler - Amella Pharma, Llc (081189492)