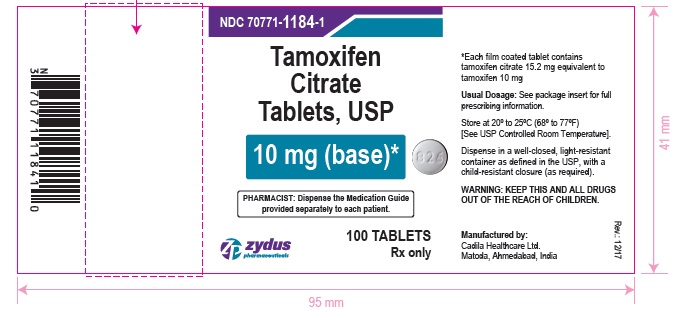
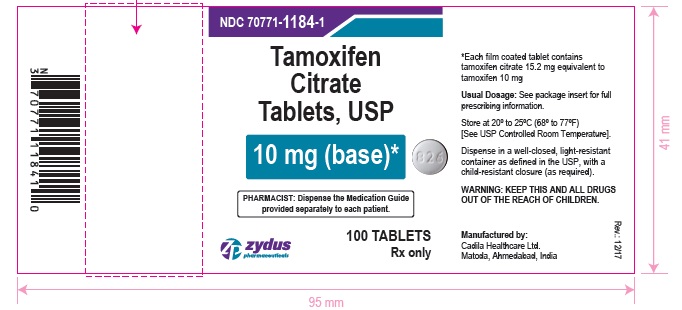
Label: TAMOXIFEN CITRATE tablet, film coated
-
NDC Code(s):
70771-1184-1,
70771-1184-6,
70771-1184-8,
70771-1184-9, view more70771-1185-0, 70771-1185-1, 70771-1185-3, 70771-1185-6, 70771-1185-9
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated September 28, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TAMOXIFEN CITRATE
tamoxifen citrate tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1184 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TAMOXIFEN CITRATE (UNII: 7FRV7310N6) (TAMOXIFEN - UNII:094ZI81Y45) TAMOXIFEN 10 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE CALCIUM (UNII: UTY7PDF93L) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score no score Shape ROUND (ROUND) Size 7mm Flavor Imprint Code 826 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1184-6 60 in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2018 2 NDC:70771-1184-9 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2018 3 NDC:70771-1184-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2018 4 NDC:70771-1184-8 180 in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206694 01/03/2018 TAMOXIFEN CITRATE
tamoxifen citrate tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1185 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TAMOXIFEN CITRATE (UNII: 7FRV7310N6) (TAMOXIFEN - UNII:094ZI81Y45) TAMOXIFEN 20 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE CALCIUM (UNII: UTY7PDF93L) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score no score Shape ROUND (ROUND) Size 9mm Flavor Imprint Code 827 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1185-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2018 2 NDC:70771-1185-6 60 in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2018 3 NDC:70771-1185-9 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2018 4 NDC:70771-1185-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2018 5 NDC:70771-1185-0 1000 in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206694 01/03/2018 Labeler - Zydus Lifesciences Limited (918596198) Registrant - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 863362789 ANALYSIS(70771-1184, 70771-1185) , MANUFACTURE(70771-1184, 70771-1185)