Label: ALPHACIDE ACTIVATOR- citric acid solution
- NDC Code(s): 33642-4190-1, 33642-4190-2, 33642-4190-3, 33642-4190-4
- Packager: Alpha Technology USA Corp
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated July 30, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
- WARNINGS AND PRECAUTIONS
- ACTIVE INGREDIENT
- SPL UNCLASSIFIED SECTION
-
OTHER SAFETY INFORMATION
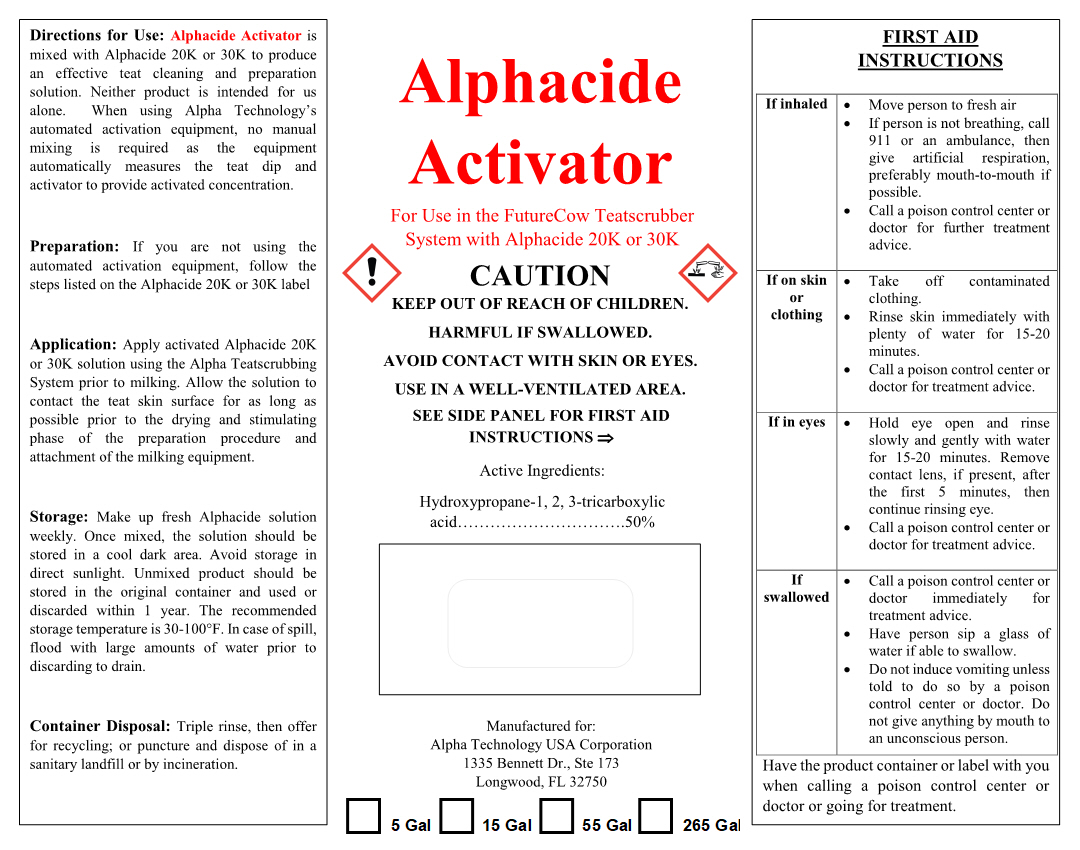
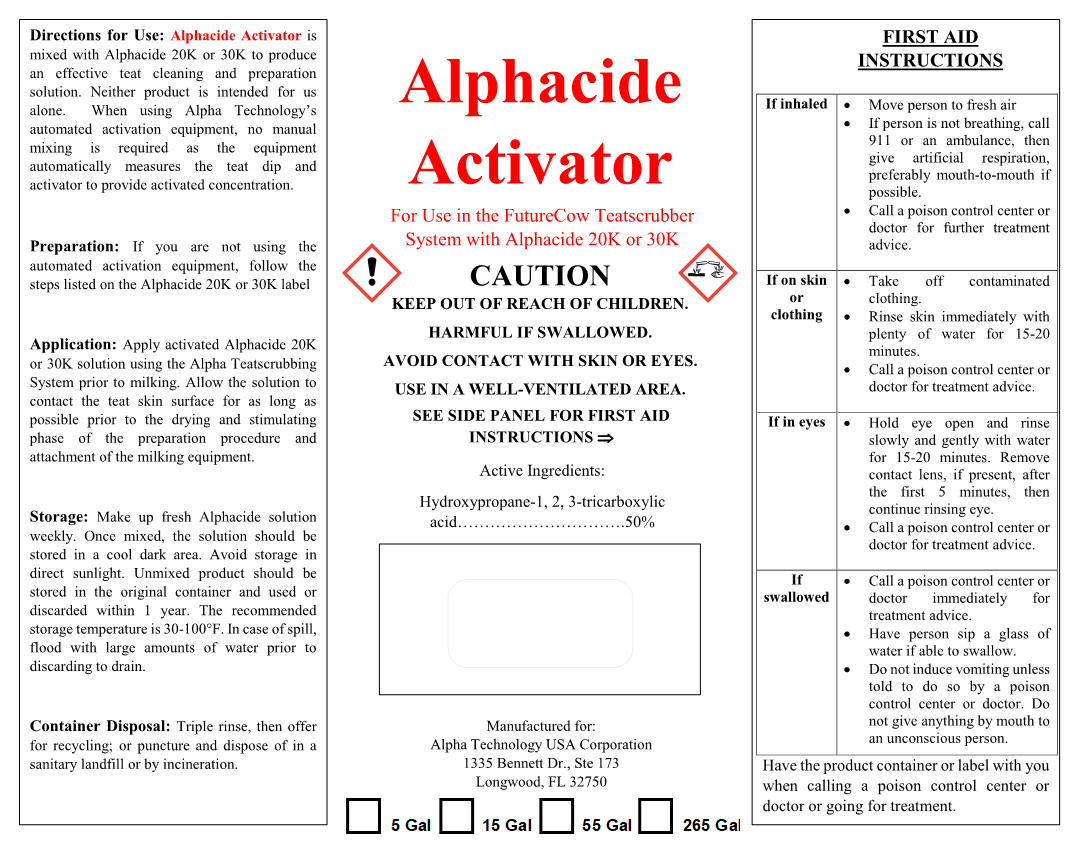
FIRST AID INSTRUCTIONS
If inhaled - Move person to fresh air. If person is not breathing, call 911 or an ambulance, then give artificial respiration, preferably mouth-to-mouth if possible. Call a poison control center or doctor for further treatment advice.
If on skin or clothing - Take off contaminated clothing. Rinse skin immediately with plenty of water for 15-20 minutes. Call a poison control center or doctor for treatment advice.
If in eyes - Hold eye open and rinse slowly and gently with water for 15-20 minutes. Remove contact lens, if present, after the first 5 minutes, then continue rinsing eye. Call a poison control center or doctor for treatment advice.
If swallowed - Call a poison control center or doctor immediately for treatment advice. Have person sip a glass of water if able to swallow . Do not induce vomiting unless told to do so by a poison control center or doctor. Do not give anything by mouth to an unconscious person.
Have the product container or label with you when calling a poison control center or doctor or going for treatment.
-
INSTRUCTIONS FOR USE
Directions for Use: Alphacide Activator is mixed with Alphacide 20K or 30K to produce an effective teat cleaning and preparation solution. Neither product is intended for us alone. When using Alpha Technology's automated activation equipment, no manual mixmg is required as the equipment automatically measures the teat dip and activator to provide activated concentration.
Preparation: If you are not using the automated activation equipment, follow the steps listed on the Alphacide 20K or 30K label
Application: Apply activated Alphacide 20K or 30K solution using the Alpha Teatscrubbing System prior to milking. Allow the solution to contact the teat skin surface for as long as possible prior to the drying and stimulating phase of the preparation procedure and attachment of the milking equipment.
Storage: Make up fresh Alphacide solution weekly. Once mixed, the solution should be stored in a cool dark area. Avoid storage in direct sunlight. Unmixed product should be stored in the original container and used or discarded within 1 year. The recommended storage temperature is 30-100°F. In case of spill, flood with large amounts of water prior to discarding to drain.
Container Disposal: Triple rinse, then offer for recycling; or puncture and dispose of in a sanitary landfill or by incineration .
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALPHACIDE ACTIVATOR
citric acid solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:33642-4190 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 0.50 kg in 1 kg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 0.50 kg in 1 kg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:33642-4190-1 19 kg in 1 CONTAINER 2 NDC:33642-4190-2 57 kg in 1 DRUM 3 NDC:33642-4190-3 208 kg in 1 DRUM 4 NDC:33642-4190-4 1040 kg in 1 CONTAINER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/29/2019 Labeler - Alpha Technology USA Corp (012557756)