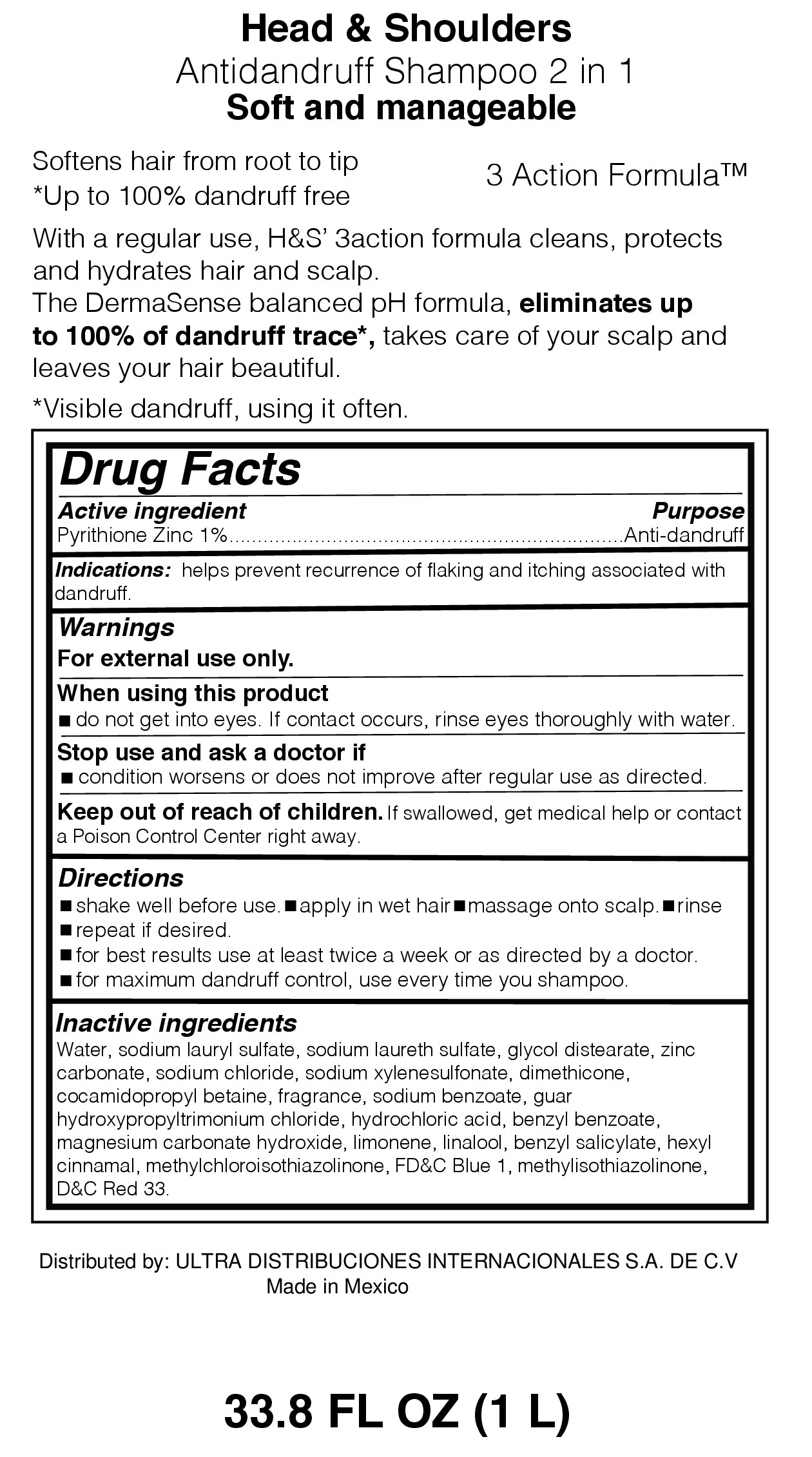
Label: HEAD AND SHOULDERS 2 IN SOFT AND MANAGEABLE- head and shoulders lotion/shampoo
-
Contains inactivated NDC Code(s)
NDC Code(s): 72389-103-01, 72389-103-02, 72389-103-03 - Packager: ULTRA DISTRIBUCIONES INTERNACIONALES, S.A. DE C.V.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 14, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Purpose
- Active Ingredient
- Warnings
- When using this product
- Stop use and ask doctor if
- Keep our of reach of children
- Directions
-
Inactive Ingredients
Water, sodium lauryl sulfate, sodium laureth sulfate, glycol distearate,
dimethicone, zinc carbonate, sodium chloride, sodium xylenesulfonate,
cocamidopropyl betaine, parfum, sodium benzoate, guar
hydroxypropyltrimonium chloride, hydrochloric acid, hexyl cinnamal,
linalool, magnesium carbonate hydroxide, methylchloroisothiazolinone,
FD&C Blue 1, methylisothiaolinone, D&C Red.33
- Distributed by:
- Indications:
- Principal Display Panel Antidandruff Shampoo 2 in 1 Soft and Manageable
-
INGREDIENTS AND APPEARANCE
HEAD AND SHOULDERS 2 IN SOFT AND MANAGEABLE
head and shoulders lotion/shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72389-103 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC 1 g in 100 mL Inactive Ingredients Ingredient Name Strength BENZYL SALICYLATE (UNII: WAO5MNK9TU) LIMONENE, (+)- (UNII: GFD7C86Q1W) D&C RED NO. 33 (UNII: 9DBA0SBB0L) WATER (UNII: 059QF0KO0R) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) GLYCOL DISTEARATE (UNII: 13W7MDN21W) LINALOOL, (-)- (UNII: 3U21E3V8I2) MAGNESIUM CARBONATE HYDROXIDE (UNII: YQO029V1L4) DIMETHICONE (UNII: 92RU3N3Y1O) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM XYLENESULFONATE (UNII: G4LZF950UR) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) SODIUM BENZOATE (UNII: OJ245FE5EU) GUAR HYDROXYPROPYLTRIMONIUM CHLORIDE (1.7 SUBSTITUENTS PER SACCHARIDE) (UNII: B16G315W7A) HYDROCHLORIC ACID (UNII: QTT17582CB) ZINC CARBONATE (UNII: EQR32Y7H0M) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) BENZYL BENZOATE (UNII: N863NB338G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72389-103-01 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/26/2018 2 NDC:72389-103-02 375 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/26/2018 3 NDC:72389-103-03 180 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/26/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 06/26/2018 Labeler - ULTRA DISTRIBUCIONES INTERNACIONALES, S.A. DE C.V. (812839648) Registrant - ULTRA DISTRIBUCIONES INTERNACIONALES, S.A. DE C.V. (815333653) Establishment Name Address ID/FEI Business Operations PROCTER & GAMBLE MANUFACTURING MEXICO, S. DE R.L. DE C.V. 812807550 manufacture(72389-103) Establishment Name Address ID/FEI Business Operations ULTRA DISTRIBUCIONES INTERNACIONALES, S.A. DE C.V. 812839648 label(72389-103)