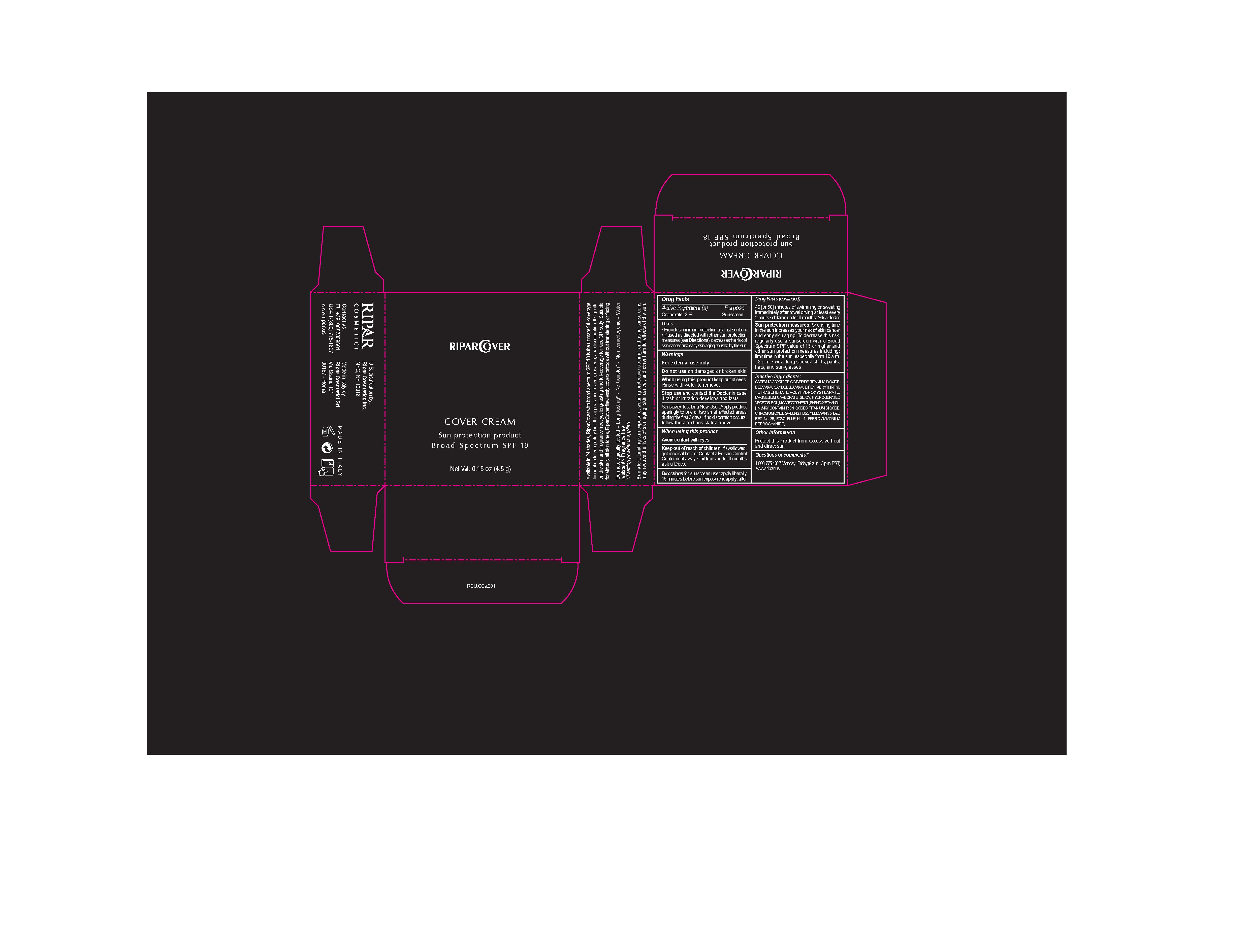
Label: COVER CREAM. SUN PROTECTION- octinoxate cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 72399-115-01, 72399-115-02 - Packager: Ripar Cosmetici srl
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 23, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS PURPOSE
-
Warnings
SUN ALERT: Limiting sun exposures, wearing protective clothing and using sunscreens may reduce the risks of skin aging. skin cancer, and other harmful effects of the sun
For external use only.
Do not use on damaged or broken skin.
When using this product keep our of the eyes. Rinse with water to remove.
SUN PROTECTION MEASURES. Spending times in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad Spectrum SPF value 15 or higher and other sun protection measures including: limit time to the sun especially from 10:00 am to 2:00 pm. Weat long sleeve shirts , pants, hats and sunglasses
DIscontinue uses if signs or irritationsor rash appears. If irritation or rash persists, consult with a doctor.
Keep out of reach of children. If swallowed, get medical help or Contact Poison Control Central right away
Children under 6 months ask a doctor
- Questions or comments?
- Other Information
- Directions
- Uses
-
Inactive Ingredients
CAPRYLIC/CAPRIC TRIGLYCERIDE, MEDIUM CHAIN TRIGLYCERIDES, BEESWAX YELLOW WAX, CANDELILLA WAX, OCTINOXATE, DIPENTAERYTHRITYL TRI-POLYHYDROXYSTEARATE, MAGNESIUM CARBONATE, SILICON DIOXIDE, HYDROGENATED PALM, MICA, TOCOPHEROL , PHENOXYETHANOL, FERRIC OXIDE YELLOW , FERRIC OXIDE RED, TITANIUM DIOXIDE, FERROSOFERRIC OXIDE
- Uses
- Package Label
-
INGREDIENTS AND APPEARANCE
COVER CREAM. SUN PROTECTION
octinoxate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72399-115 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength FERROSOFERRIC OXIDE (UNII: XM0M87F357) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) YELLOW WAX (UNII: 2ZA36H0S2V) CANDELILLA WAX (UNII: WL0328HX19) DIPENTAERYTHRITYL TRI-POLYHYDROXYSTEARATE (UNII: D21K655H52) MAGNESIUM CARBONATE (UNII: 0E53J927NA) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYDROGENATED PALM OIL (UNII: 257THB963H) MICA (UNII: V8A1AW0880) TOCOPHEROL (UNII: R0ZB2556P8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72399-115-02 1 in 1 BOX 06/27/2018 1 NDC:72399-115-01 4 mL in 1 CYLINDER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/27/2018 Labeler - Ripar Cosmetici srl (338675564) Registrant - Ripar Cosmetici srl (338675564) Establishment Name Address ID/FEI Business Operations 338675564 338675564 manufacture(72399-115)