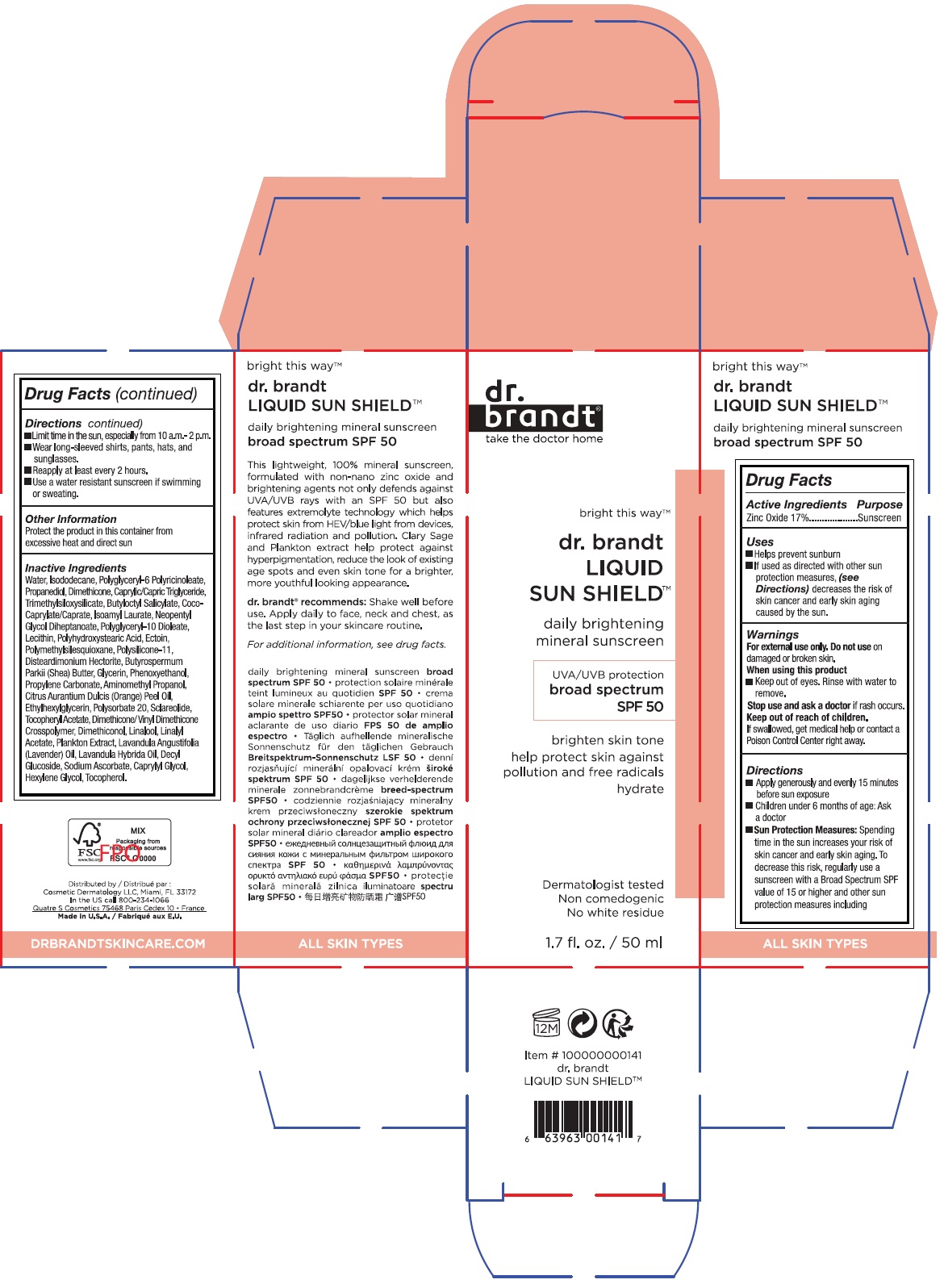
Label: DR. BRANDT SUN SHIELD SPF 50- zinc oxide liquid
- NDC Code(s): 81568-101-50
- Packager: Dr. Brandt
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 15, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
- Apply generously and evenly 15 minutes before sun exposure
- Children under 6 months of age: Ask a doctor
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats and sunglasses.
- Reapply at least every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
- Other Information
-
Inactive Ingredients
Water, Isododecane, Polyglyceryl-6 Polyricinoleate, Propanediol, Dimethicone, Caprylic/Capric Triglyceride, Trimethylsiloxysilicate, Butyloctyl Salicylate, Coco-Caprylate/Caprate, Isoamyl Laurate, Neopentyl Glycol Diheptanoate, Polyglyceryl-10 Dioleate, Lecithin, Polyhydroxystearic Acid, Ectoin, Polymethylsilsesquioxane, Polysilicone-11, Disteardimonium Hectorite, Butyrospermum Parkii (Shea) Butter, Glycerin, Phenoxyethanol, Propylene Carbonate, Aminomethyl Propanol, Citrus Aurantium Dulcis (Orange) Peel Oil, Ethylhexylglycerin, Polysorbate 20, Sclareolide, Tocopheryl Acetate, Dimethicone/Vinyl Dimethicone Crosspolymer, Dimethiconol, Linalool, Linalyl Acetate, Plankton Extract, Lavandula Angustifolia (Lavender) Oil, Lavandula Hybrida Oil, Decyl Glucoside, Sodium Ascorbate, Caprylyl Glycol, Hexylene Glycol, Tocopherol
-
SPL UNCLASSIFIED SECTION
take the doctor home
bright this wayTM
daily brightening mineral sunscreen
UVA/UVB protection
broad spectrum SPF 50
brighten skin tone
help protect skin against pollution and free radicals
hydrate
Dermatologist tested
Non comedogenic
No white residue
This lightweight, 100% mineral sunscreen, formulated with non-nano zinc oxide and brightening agents not only defends against UVA/UVB rays with an SPF 50 but also features extremolyte technology which helps protect skin from HEV/blue light from devices, infrared radiation and pollution. Clary Sage and Plankton extract help protect against hyperpigmentation, reduce the look of existing age spots and even skin tone for a brighter, more youthful looking appearance.
dr. brand® recommends: Shake well before use. Apply daily to face, neck and chest, as the last step in your skincare routine.
ALL SKIN TYPES
Distributed by :
Cosmetic Dermatology LLC, Miami, FL 33172
In the US call 800-234-1066
Quatre S Cosmetics 75468 Paris Cedex 10 • France
Made in U.S.A.
DRBRANDTSKINCARE.COM
- Packaging
-
INGREDIENTS AND APPEARANCE
DR. BRANDT SUN SHIELD SPF 50
zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81568-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 17 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISODODECANE (UNII: A8289P68Y2) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) PROPANEDIOL (UNII: 5965N8W85T) DIMETHICONE (UNII: 92RU3N3Y1O) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) COCOYL CAPRYLOCAPRATE (UNII: 8D9H4QU99H) ISOAMYL LAURATE (UNII: M1SLX00M3M) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) POLYGLYCERYL-10 DIPALMITATE (UNII: 84VD0IA2S6) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) ECTOINE (UNII: 7GXZ3858RY) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) SHEA BUTTER (UNII: K49155WL9Y) GLYCERIN (UNII: PDC6A3C0OX) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLENE CARBONATE (UNII: 8D08K3S51E) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) ORANGE OIL (UNII: AKN3KSD11B) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) POLYSORBATE 20 (UNII: 7T1F30V5YH) SCLAREOLIDE (UNII: 37W4O0O6E6) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) DIMETHICONOL (600000 CST) (UNII: RKI3S914RT) LINALOOL, (+/-)- (UNII: D81QY6I88E) LINALYL ACETATE (UNII: 5K47SSQ51G) CHLORELLA VULGARIS (UNII: RYQ4R60M02) LAVENDER OIL (UNII: ZBP1YXW0H8) LAVANDIN OIL (UNII: 9RES347CKG) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) SODIUM ASCORBATE (UNII: S033EH8359) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYLENE GLYCOL (UNII: KEH0A3F75J) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81568-101-50 1 in 1 BOX 02/15/2021 1 50 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 02/15/2021 Labeler - Dr. Brandt (361999386)