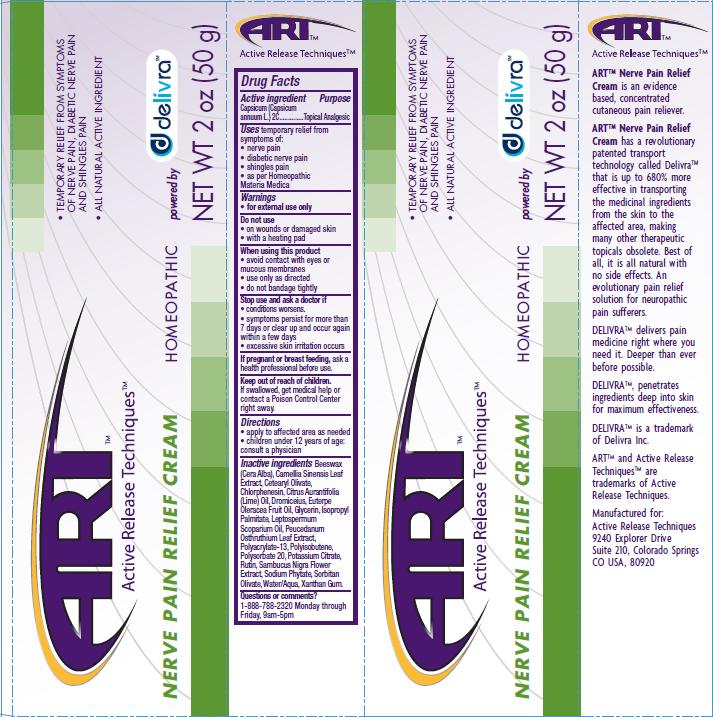
Label: NERVE PAIN RELIEF- capsicum cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 76029-002-02 - Packager: Active Release Techniques
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated March 30, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Beeswax (Cera Alba), Camellia Sinensis Leaf Extract, Cetearyl Olivate, Chlrophenesin, Citrus Aurantifolia (Lime) Oil, Dromiceius, Euterpe Oleracea Fruit Oil, Glycerin, Isopropyl Palmitate, Leptospermum Scoparium Oil, Peucedanum Osthruthium Leaf Extract, Polyacrylate-13, Polyisobutene, Polysorbate 20, Potassium Citrate, Rutin, Sambucus Nigra Flower Extract, Sodium Phytate, Sorbitan Olivate, Water/Aqua, Xanthan Gum
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NERVE PAIN RELIEF
capsicum creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76029-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSICUM (UNII: 00UK7646FG) (CAPSICUM - UNII:00UK7646FG) CAPSICUM 2 [hp_C] in 50 g Inactive Ingredients Ingredient Name Strength WHITE WAX (UNII: 7G1J5DA97F) BANCHA TEA LEAF/TWIG (UNII: EWI42IEH1C) CETEARYL OLIVATE (UNII: 58B69Q84JO) CHLORPHENESIN (UNII: I670DAL4SZ) CITRUS AURANTIIFOLIA LEAF OIL (UNII: D8XVD1D0MQ) ACAI OIL (UNII: Z0W6766A2W) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) MANUKA OIL (UNII: M6QU9ZUH2X) PEUCEDANUM OSTRUTHIUM LEAF (UNII: 86P27YRR6Y) POLYSORBATE 20 (UNII: 7T1F30V5YH) POTASSIUM CITRATE (UNII: EE90ONI6FF) RUTIN (UNII: 5G06TVY3R7) SAMBUCUS NIGRA FLOWER (UNII: 07V4DX094T) SORBITAN OLIVATE (UNII: MDL271E3GR) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76029-002-02 50 g in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/20/2011 Labeler - Active Release Techniques (174462593) Registrant - Active Release Techniques (174462593) Establishment Name Address ID/FEI Business Operations Leo Desilets maitre herboriste Inc/Facotek Packaging 245423439 manufacture