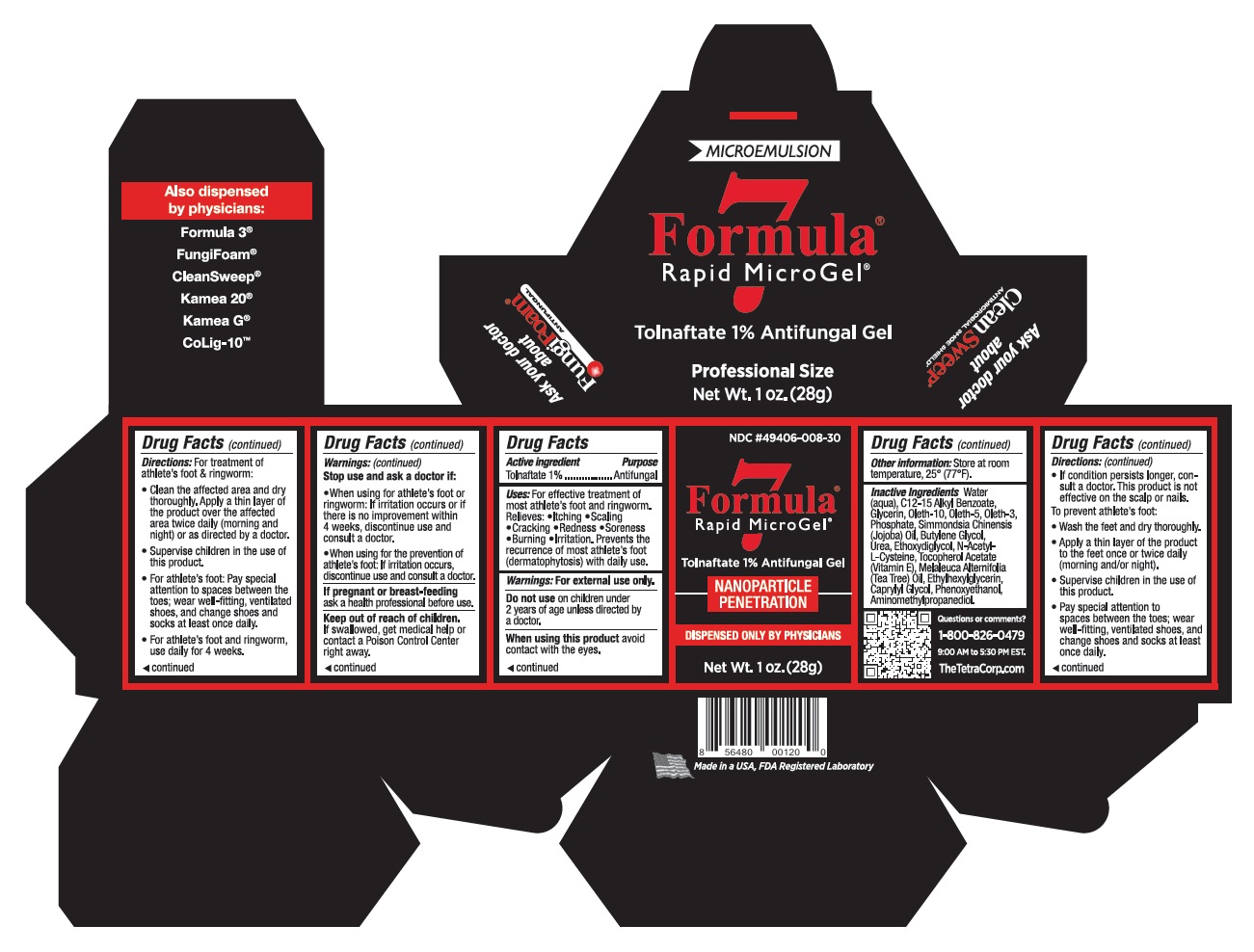
Label: FORMULA 7 THEGEL- tolnaftate gel
- NDC Code(s): 49406-008-28, 49406-008-30
- Packager: The Tetra Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Uses:
- INDICATIONS & USAGE
- Active ingredient
- Stop use and ask a doctor if:
- DO NOT USE SECTION
- PREGNANCY OR BREAST FEEDING
- Questions or comments?
-
Inactive Ingredients
Inactive Ingredients: Water (aqua),C12-15 Alkyl Benzoate, SimmondsiaChinensis (Jojoba) Oil, Glycerin,Oleth-10, Oleth-5, Oleth-3 Phosphate,PEG-20 Hydrogenated Lanolin,Butylene Glycol, Urea, Ethoxydiglycol,N-Acetyl-L-Cysteine, TocopherolAcetate (Vitamin E), Melaleuca
Alternifolia (Tea Tree) Oil, DisodiumEDTA, Ethylhexylglycerin, CaprylylGlycol, Phenoxyethanol, Aminomethylpropanediol,Sodium Chloride. -
Directions:
For treatment of athlete’s foot and ringworm:
• Clean the affected area and dry thoroughly. Apply a thin layer of the product over the affected area twice daily (morning and night) or as
directed by a doctor.
• Supervise children in the use of this product.
• For athlete’s foot: Pay special attention to spaces between the toes; wear well-fitting, ventilated shoes, and change shoes and socks
at least once daily.
• For athlete’s foot and ringworm, use daily for 4 weeks.
• If condition persists longer, consult a doctor. This product is not effective on the scalp or nails.To prevent athlete’s foot:
• Wash the feet and dry thoroughly.
• Apply a thin layer of the product to the feet once or twice daily (morning and/or night).
• Supervise children in the use of this product.
• Pay special attention to spaces between the toes; wear well-fitting,ventilated shoes, and change shoes and socks at least once daily. - Other information
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Product Label
-
INGREDIENTS AND APPEARANCE
FORMULA 7 THEGEL
tolnaftate gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49406-008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 1 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) JOJOBA OIL (UNII: 724GKU717M) GLYCERIN (UNII: PDC6A3C0OX) OLETH-10 (UNII: JD797EF70J) OLETH-5 (UNII: 1GH33785AY) OLETH-3 PHOSPHATE (UNII: 8Q0Z18J1VL) PEG-20 HYDROGENATED LANOLIN (UNII: 5PP3KJ4T6S) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) UREA (UNII: 8W8T17847W) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TEA TREE OIL (UNII: VIF565UC2G) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHENOXYETHANOL (UNII: HIE492ZZ3T) ACETYLCYSTEINE (UNII: WYQ7N0BPYC) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49406-008-30 15 g in 1 PACKAGE; Type 0: Not a Combination Product 06/13/2018 2 NDC:49406-008-28 28 g in 1 JAR; Type 0: Not a Combination Product 01/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 06/13/2018 Labeler - The Tetra Corporation (829958409) Registrant - The Tetra Corporation (829958409)