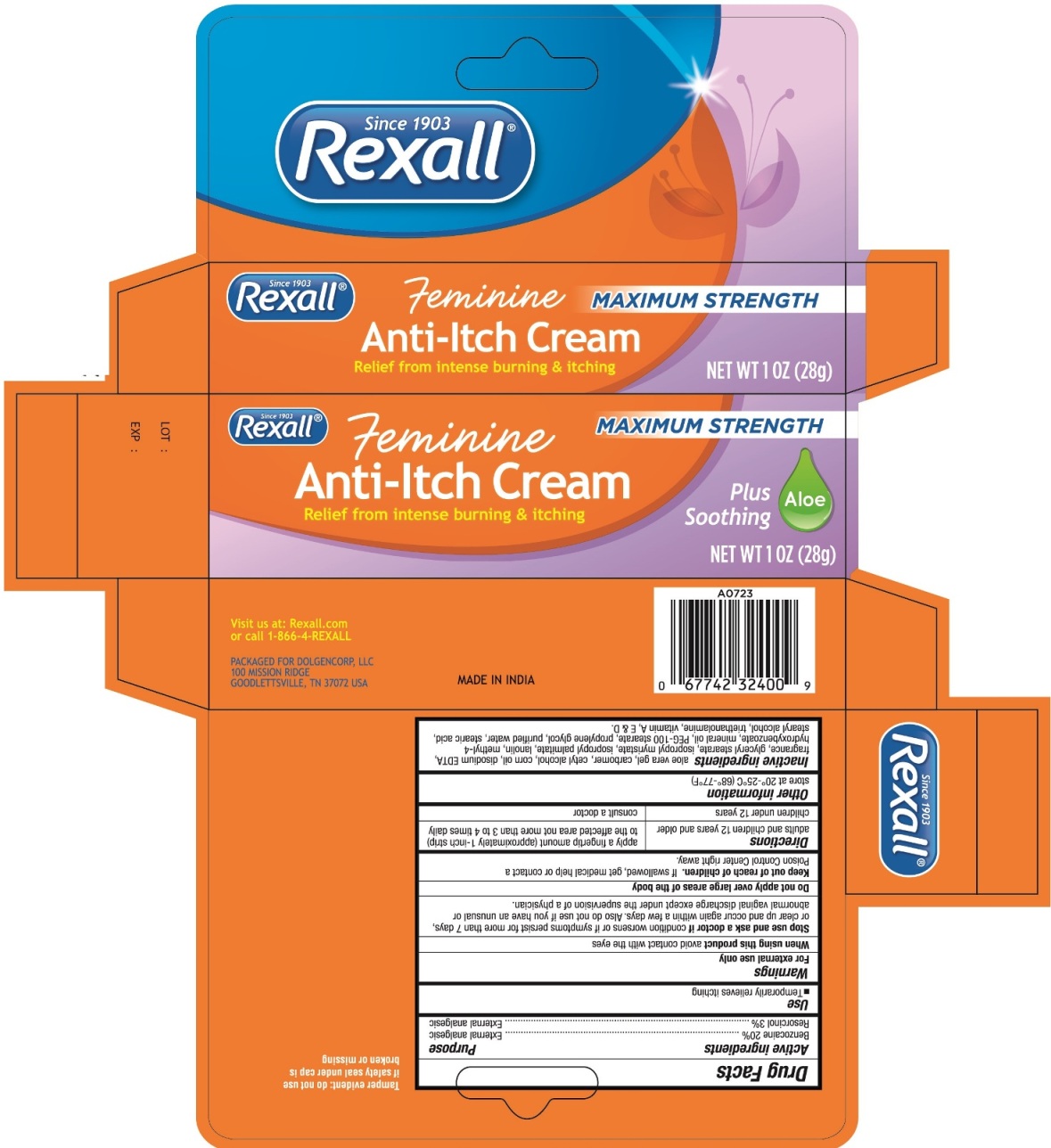
Label: REXALL FEMININE MAXIMUM STRENGTH ANTI-ITCH- benzocaine and resorcinol cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 55910-415-03 - Packager: Dolgencorp, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 28, 2016
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Use
-
Warnings
For external use only
- Directions
- Other information
-
Inactive ingredients
aloe vera gel, carbomer, cetyl alcohol, corn oil, disodium EDTA, fragrance, glyceryl stearate, isopropyl myristate, isopropyl palmitate, lanolin, methyl-4 hydroxybenzoate, mineral oil, PEG-100 stearate, propylene glycol, purified water, stearic acid, stearyl alcohol, triethanolamine, vitamin A, E & D
- Principal display panel - 28 g Carton Label
-
INGREDIENTS AND APPEARANCE
REXALL FEMININE MAXIMUM STRENGTH ANTI-ITCH
benzocaine and resorcinol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55910-415 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 20 g in 100 g RESORCINOL (UNII: YUL4LO94HK) (RESORCINOL - UNII:YUL4LO94HK) RESORCINOL 3 g in 100 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) CETYL ALCOHOL (UNII: 936JST6JCN) CORN OIL (UNII: 8470G57WFM) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) LANOLIN (UNII: 7EV65EAW6H) METHYLPARABEN (UNII: A2I8C7HI9T) MINERAL OIL (UNII: T5L8T28FGP) PEG-100 STEARATE (UNII: YD01N1999R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) STEARIC ACID (UNII: 4ELV7Z65AP) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) TROLAMINE (UNII: 9O3K93S3TK) VITAMIN A (UNII: 81G40H8B0T) VITAMIN D (UNII: 9VU1KI44GP) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55910-415-03 28 g in 1 TUBE; Type 0: Not a Combination Product 09/27/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part346 09/27/2016 Labeler - Dolgencorp, Inc. (068331990) Registrant - Anicare Pharmaceuticals Pvt. Ltd (916837425) Establishment Name Address ID/FEI Business Operations Anicare Pharmaceuticals Pvt. Ltd 916837425 manufacture(55910-415)