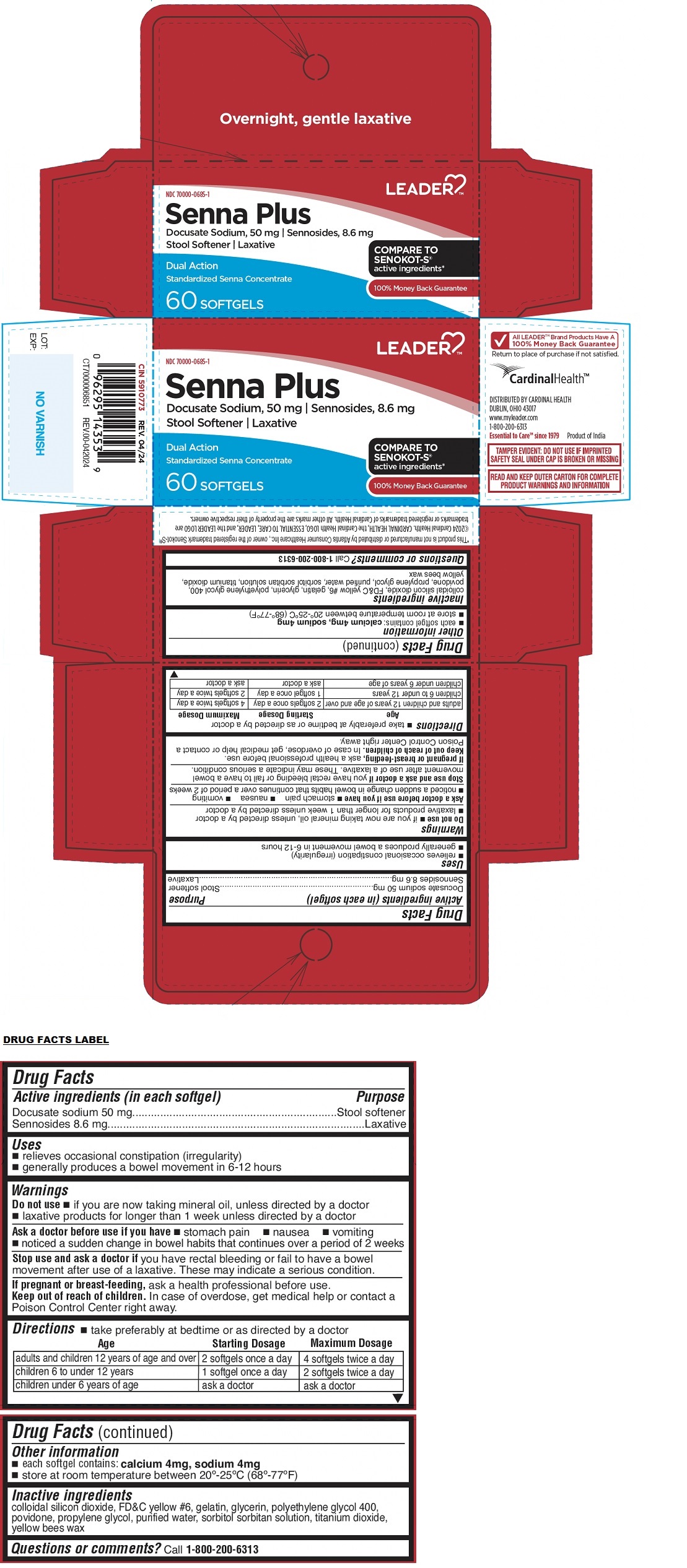
Label: LEADER SENNA PLUS STOOL SOFTENER LAXATIVE SOFTGEL- docusate sodium, sennosides capsule, liquid filled
- NDC Code(s): 70000-0685-1
- Packager: Cardinal Health 110, LLC. DBA Leader
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients (in each softgel)
- Purpose
- Uses
-
Warnings
Do not use • if you are now taking mineral oil, unless directed by a doctor • laxative products for longer than 1 week unless directed by a doctor
Ask a doctor before use if you have • stomach pain • nausea • vomiting • noticed a sudden change in bowel habits that continues over a period of 2 weeks
Stop use and ask a doctor if you have rectal bleeding or fail to have a bowel movement after use of a laxative. These may indicate a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
-
Directions
• take preferably at bedtime or as directed by a doctor
Age Starting Dosage Maximum Dosage adults and children 12 years of age and over 2 softgels once a day 4 softgels twice a day children 6 to under 12 years 1 softgel once a day 2 softgels twice a day children under 6 years of age ask a doctor ask a doctor - Other information
- Inactive ingredients
- Questions or comments?
-
SPL UNCLASSIFIED SECTION
COMPARE TO SENOKOT-S® active ingredients*
Dual Action
Standardized Senna Concentrate
All LEADER™ Brand Products Have A 100% Money Back Guarantee
Return to place of purchase if not satisfied.CardinalHealth™
DISTRIBUTED BY CARDINAL HEALTH
DUBLIN, OHIO 43017
www.myleader.com
1-800-200-6313
Essential to Care™ Since 1979Product of India
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
READ AND KEEP OUTER CARTON FOR COMPLETE PRODUCT WARNINGS AND INFORMATION
Overnight, gentle laxative
*This product is not manufactured or distributed by Atlantis Consumer Healthcare Inc., owner of the registered trademark Senokot-S®
©2024 Cardinal Health. CARDINAL HEALTH, the Cardinal Health LOGO, ESSENTIAL TO CARE, LEADER, and the LEADER LOGO are trademarks or registered trademarks of Cardinal Health. All other marks are the property of their respective owners.
- Packaging
-
INGREDIENTS AND APPEARANCE
LEADER SENNA PLUS STOOL SOFTENER LAXATIVE SOFTGEL
docusate sodium, sennosides capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70000-0685 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) SORBITAN (UNII: 6O92ICV9RU) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) YELLOW WAX (UNII: 2ZA36H0S2V) Product Characteristics Color orange Score no score Shape OVAL Size 12mm Flavor Imprint Code 902 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70000-0685-1 1 in 1 CARTON 07/16/2024 1 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 07/16/2024 Labeler - Cardinal Health 110, LLC. DBA Leader (063997360)