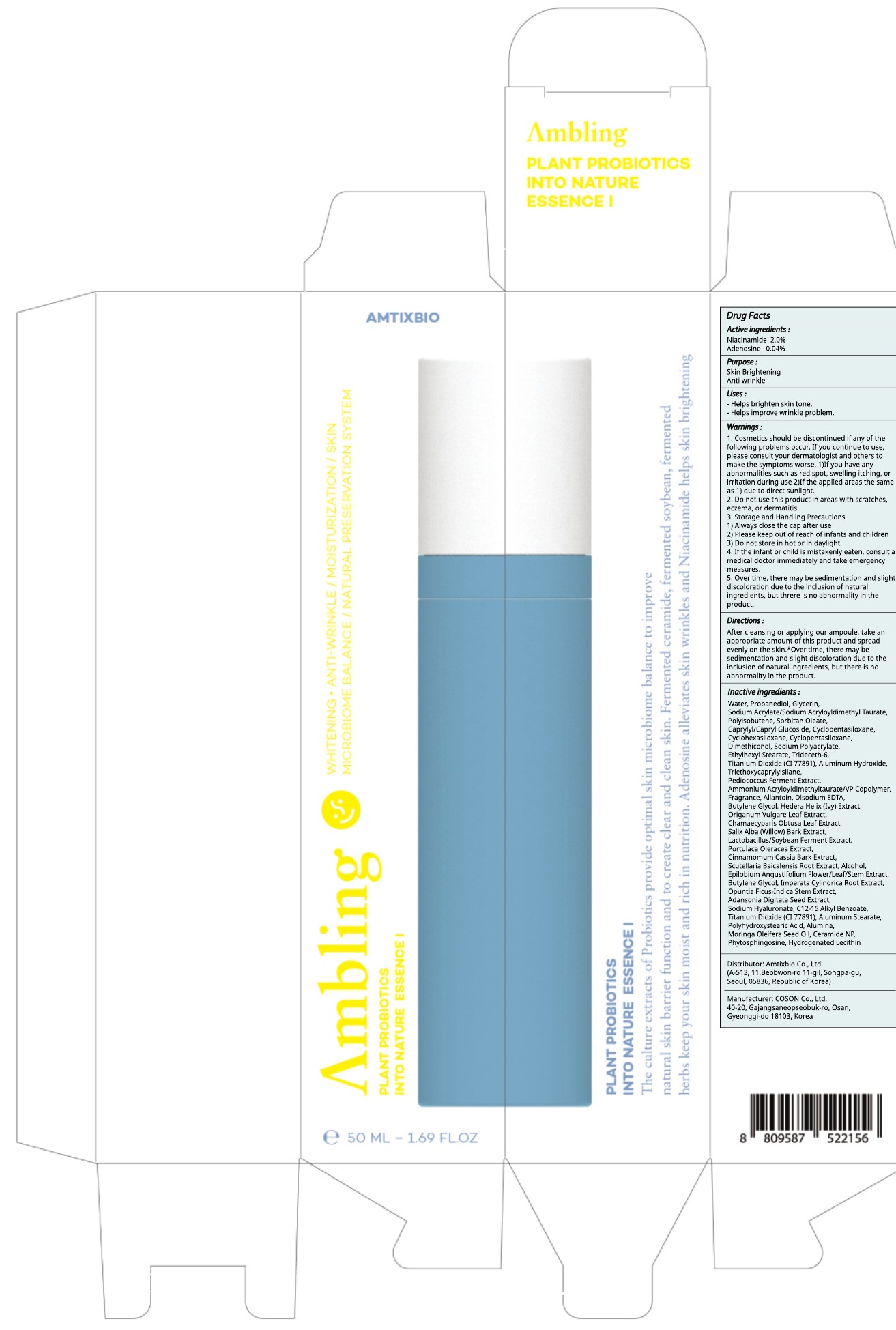
Label: AMBLING PLANT PROBIOTICS INTO NATURE ESSENCE I- niacinamide, adenosine liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 73456-030-01, 73456-030-02 - Packager: Amtixbio Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 2, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive ingredients:
Water, Propanediol, Glycerin, Sodium Acrylate/Sodium Acryloyldimethyl Taurate, Polyisobutene, Sorbitan Oleate, Caprylyl/Capryl Glucoside, Cyclopentasiloxane, Cyclohexasiloxane, Cyclopentasiloxane, Dimethiconol, Sodium Polyacrylate, Ethylhexyl Stearate, Trideceth-6, Titanium Dioxide (CI 77891), Aluminum Hydroxide, Triethoxycaprylylsilane, Pediococcus Ferment Extract, Ammonium Acryloyldimethyltaurate/VP Copolymer, Fragrance, Allantoin, Disodium EDTA, Butylene Glycol, Hedera Helix (Ivy) Extract, Origanum Vulgare Leaf Extract, Chamaecyparis Obtusa Leaf Extract, Salix Alba (Willow) Bark Extract, Lactobacillus/Soybean Ferment Extract, Portulaca Oleracea Extract, Cinnamomum Cassia Bark Extract, Scutellaria Baicalensis Root Extract, Alcohol, Epilobium Angustifolium Flower/Leaf/Stem Extract, Butylene Glycol, Imperata Cylindrica Root Extract, Opuntia Ficus-Indica Stem Extract, Adansonia Digitata Seed Extract, Sodium Hyaluronate, C12-15 Alkyl Benzoate, Titanium Dioxide (CI 77891), Aluminum Stearate, Polyhydroxystearic Acid, Alumina, Moringa Oleifera Seed Oil, Ceramide NP, Phytosphingosine, Hydrogenated Lecithin
- PURPOSE
-
WARNINGS
Warnings:
1. Cosmetics should be discontinued if any of the following problems occur. If you continue to use, please consult your dermatologist and others to make the symptoms worse. 1)If you have any abnormalities such as red spot, swelling itching, or irritation during use 2)If the applied areas the same as 1) due to direct sunlight.
2. Do not use this product in areas with scratches, eczema, or dermatitis.
3. Storage and Handling Precautions
1) Always close the cap after use
2) Please keep out of reach of infants and children
3) Do not store in hot or in daylight.
4. If the infant or child is mistakenly eaten, consult a medical doctor immediately and take emergency measures.
5. Over time, there may be sedimentation and slight discoloration due to the inclusion of natural ingredients, but threre is no abnormality inthe product. - KEEP OUT OF REACH OF CHILDREN
- Uses
- Directions
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AMBLING PLANT PROBIOTICS INTO NATURE ESSENCE I
niacinamide, adenosine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73456-030 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Niacinamide (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) Niacinamide 1.00 g in 50 mL Adenosine (UNII: K72T3FS567) (ADENOSINE - UNII:K72T3FS567) Adenosine 0.02 g in 50 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Propanediol (UNII: 5965N8W85T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73456-030-02 1 in 1 CARTON 10/01/2019 1 NDC:73456-030-01 50 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/01/2019 Labeler - Amtixbio Co., Ltd. (694464882) Registrant - Amtixbio Co., Ltd. (694464882) Establishment Name Address ID/FEI Business Operations COSON Co., Ltd._Osan Plant 689847210 manufacture(73456-030)