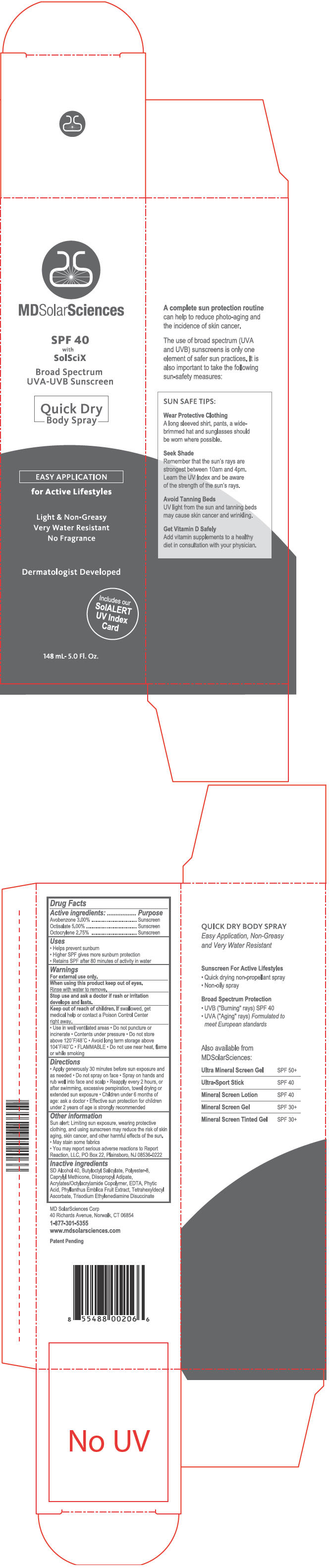
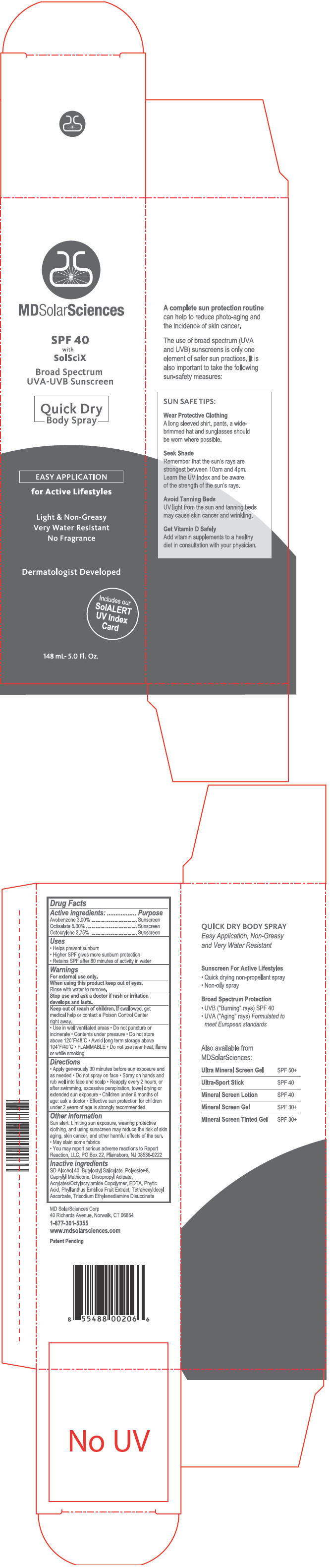
Label: MDSOLARSCIENCES SPF 40 QUICK DRY- avobenzone, octisalate, and octocrylene spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 49358-544-01 - Packager: MD Solar Sciences
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 24, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- STORAGE AND HANDLING
-
Directions
- Apply generously 30 minutes before sun exposure and as needed
- Do not spray on face
- Spray on hands and rub well into face and scalp
- Reapply every 2 hours, or after swimming, excessive perspiration, towel drying or extended sun exposure
- Children under 6 months of age: ask a doctor
- Effective sun protection for children under 2 years of age is strongly recommended
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL - 148 mL Can Carton
-
INGREDIENTS AND APPEARANCE
MDSOLARSCIENCES SPF 40 QUICK DRY
avobenzone, octisalate, and octocrylene sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49358-544 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 4.44 mL in 148 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 7.4 mL in 148 mL Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 4.07 mL in 148 mL Inactive Ingredients Ingredient Name Strength Butyloctyl Salicylate (UNII: 2EH13UN8D3) Diisopropyl Adipate (UNII: P7E6YFV72X) Edetic Acid (UNII: 9G34HU7RV0) Fytic Acid (UNII: 7IGF0S7R8I) Phyllanthus Emblica Fruit (UNII: YLX4CW2576) Tetrahexyldecyl Ascorbate (UNII: 9LBV3F07AZ) Trisodium Ethylenediamine Disuccinate (UNII: YA22H34H9Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49358-544-01 1 in 1 CARTON 1 148 mL in 1 CAN Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 02/01/2012 Labeler - MD Solar Sciences (013647301)