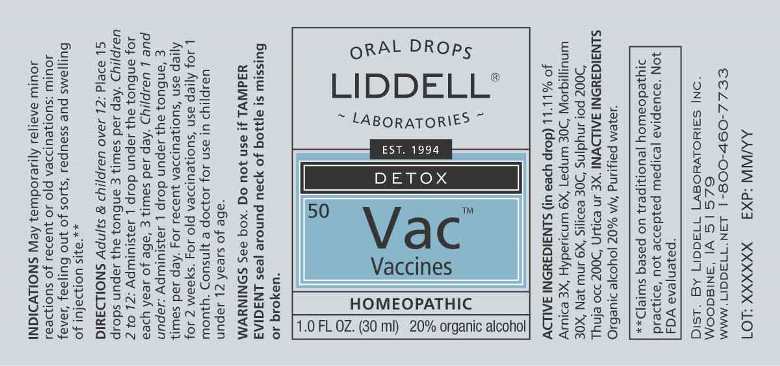
Label: VACCINES- arnica montana, hypericum perforatum, ledum palustre, morbillinum, natrum muriaticum, silicea, sulphur iodatum, thuja occidentalis, urtica urens liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 50845-0262-2 - Packager: Liddell Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 20, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS:
-
USES:
May temporarily relieve the ill effects - not the therapeutic effects - of oral or injected vaccines. Children and adults may expect temporary relief from symptoms such as:
• minor fever
• pain
• redness
• weakness
• lack of energy
• swelling**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
-
WARNINGS:
Do not use if you have ever had an allergic reaction to this product or any of its ingredients.
Ask a doctor before use if excessive redness or swelling is present.
Stop use and ask a doctor if symptoms persist for more than 7 days, worsen, or if new symptoms occur.
Keep out of reach of children. In case of overdose, get medical help or call a Poison Control Center right away.
If pregnant or breast feeding, ask a doctor before using product.
Do not use if TAMPER EVIDENT seal around neck of bottle is missing or broken.
Other Information:
Store at room temperature.
- KEEP OUT OF REACH OF CHILDREN:
-
DIRECTIONS:
Adults & children over 12: Place 15 drops under the tongue 3 times per day.
Children 2 to 12: Administer 1 drop under the tongue per year of age, 3 times per day.
Children 1 and under: Administer 1 drop under the tongue, 3 times per day.
For recent vaccinations, use daily for two weeks. For old vaccinations, use daily for one month. Consult a physician for use in children under 12.
-
INDICATIONS:
May temporarily relieve the ill effects - not the therapeutic effects - of oral or injected vaccines. Children and adults may expect temporary relief from symptoms such as:
• minor fever
• pain
• redness
• weakness
• lack of energy
• swelling**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
VACCINES
arnica montana, hypericum perforatum, ledum palustre, morbillinum, natrum muriaticum, silicea, sulphur iodatum, thuja occidentalis, urtica urens liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50845-0262 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA WHOLE (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA WHOLE 3 [hp_X] in 1 mL HYPERICUM PERFORATUM WHOLE (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM WHOLE 6 [hp_X] in 1 mL RHODODENDRON TOMENTOSUM LEAFY TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) RHODODENDRON TOMENTOSUM LEAFY TWIG 30 [hp_C] in 1 mL MEASLES VIRUS (UNII: HT3R7C012Q) (MEASLES VIRUS - UNII:HT3R7C012Q) MEASLES VIRUS 30 [hp_X] in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 6 [hp_X] in 1 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 30 [hp_C] in 1 mL SULFUR IODIDE (UNII: L6L8KA2AA0) (SULFUR IODIDE - UNII:L6L8KA2AA0) SULFUR IODIDE 300 [hp_C] in 1 mL THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 200 [hp_C] in 1 mL URTICA URENS WHOLE (UNII: IHN2NQ5OF9) (URTICA URENS - UNII:IHN2NQ5OF9) URTICA URENS WHOLE 3 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50845-0262-2 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 08/06/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/06/2020 Labeler - Liddell Laboratories, Inc. (832264241) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(50845-0262) , api manufacture(50845-0262) , label(50845-0262) , pack(50845-0262)