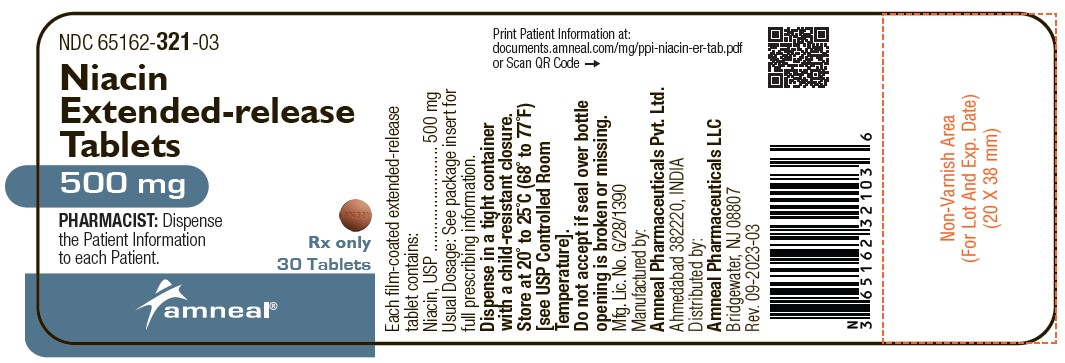
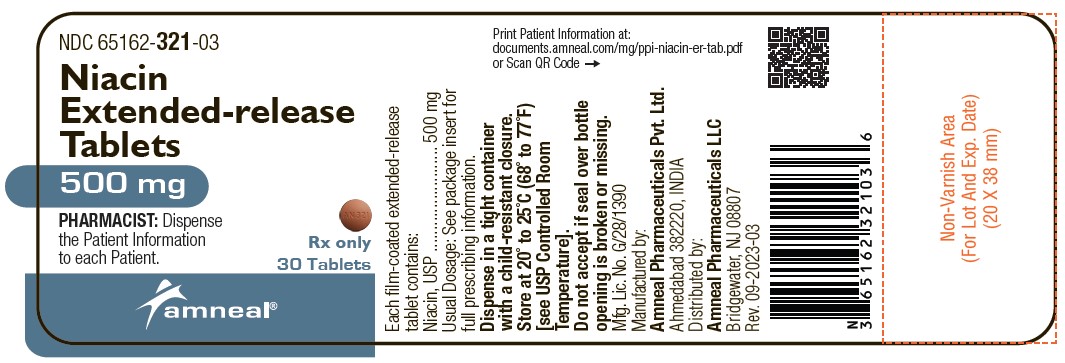
Label: NIACIN tablet, extended release
-
NDC Code(s):
65162-321-03,
65162-321-09,
65162-321-50,
65162-323-03, view more65162-323-09, 65162-323-50
- Packager: Amneal Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated September 14, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NIACIN EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for NIACIN EXTENDED-RELEASE TABLETS.
NIACIN extended-release tablets, for oral use
Initial U.S. Approval: 1997INDICATIONS AND USAGE
Niacin extended-release tablets contain extended-release niacin (nicotinic acid) and are indicated:
- To reduce elevated TC, LDL-C, Apo B and TG, and to increase HDL-C in patients with primary hyperlipidemia and mixed dyslipidemia. (1)
- To reduce the risk of recurrent nonfatal myocardial infarction in patients with a history of myocardial infarction and hyperlipidemia. (1)
- In combination with a bile acid binding resin:
- Slows progression or promotes regression of atherosclerotic disease in patients with a history of coronary artery disease (CAD) and hyperlipidemia. (1)
- As an adjunct to diet to reduce elevated TC and LDL-C in adult patients with primary hyperlipidemia. (1)
- To reduce TG in adult patients with severe hypertriglyceridemia. (1)
Limitations of use:
Addition of niacin extended-release tablets did not reduce cardiovascular morbidity or mortality among patients treated with simvastatin in a large, randomized controlled trial. (5.1)
DOSAGE AND ADMINISTRATION
- Niacin extended-release tablets should be taken at bedtime with a low-fat snack. (2.1)
- Dose range: 500 mg to 2,000 mg once daily. (2.1)
- Therapy with niacin extended-release tablets must be initiated at 500 mg at bedtime in order to reduce the incidence and severity of side effects which may occur during early therapy and should not be increased by more than 500 mg in any 4-week period. (2.1)
- Maintenance dose: 1,000 to 2,000 mg once daily. (2.2)
- Doses greater than 2,000 mg daily are not recommended. (2.2)
DOSAGE FORMS AND STRENGTHS
Unscored film-coated tablets for oral administration: 500 mg and 1,000 mg niacin extended-release. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Severe hepatic toxicity has occurred in patients substituting sustained-release niacin for immediate-release niacin at equivalent doses. (5.3)
- Myopathy has been reported in patients taking niacin extended-release. The risk for myopathy and rhabdomyolysis are increased among elderly patients; patients with diabetes, renal failure or uncontrolled hypothyroidism; and patients being treated with a statin. (5.2)
- Liver enzyme abnormalities and monitoring: Persistent elevations in hepatic transaminase can occur. Monitor liver enzymes before and during treatment. (5.3)
- Use with caution in patients with unstable angina or in the acute phase of an MI. (5)
- Niacin extended-release can increase serum glucose levels. Glucose levels should be closely monitored in diabetic or potentially diabetic patients particularly during the first few months of use or dose adjustment. (5.4)
ADVERSE REACTIONS
Most common adverse reactions (incidence > 5% and greater than placebo) are flushing, diarrhea, nausea, vomiting, increased cough, and pruritus. (6.1)
Flushing of the skin may be reduced in frequency or severity by pretreatment with aspirin (up to the recommended dose of 325 mg taken 30 minutes prior to niacin extended-release dose). (2.2)
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Statins: Caution should be used when prescribing niacin with statins as these agents can increase risk of myopathy/rhabdomyolysis. (5.2, 7.1)
- Bile Acid Sequestrants: Bile acid sequestrants have a high niacin-binding capacity and should be taken at least 4 to 6 hours before niacin extended-release administration. (7.2)
USE IN SPECIFIC POPULATIONS
- Pregnancy: Discontinue in patients with hyperlipidemia; assess individual risks and benefits in patients with hypertriglyceridemia. (8.1)
- Lactation: Advise patients not to breastfeed during treatment. (8.2)
- Renal impairment: Niacin extended-release should be used with caution in patients with renal impairment. (5, 8.6)
- Hepatic impairment: Niacin extended-release is contraindicated in active liver disease or significant or unexplained hepatic dysfunction or unexplained elevations of serum transaminases. (4,5, 5.3, 8.7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Initial Dosing
2.2 Maintenance Dose
2.3 Dosage in Patients with Renal or Hepatic Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Mortality and Coronary Heart Disease Morbidity
5.2 Skeletal Muscle
5.3 Liver Dysfunction
5.4 Laboratory Abnormalities
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Post-marketing Experience
7 DRUG INTERACTIONS
7.1 Statins
7.2 Bile Acid Sequestrants
7.3 Aspirin
7.4 Antihypertensive Therapy
7.5 Other
7.6 Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
8.8 Gender
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis and Mutagenesis and Impairment of Fertility
14 CLINICAL STUDIES
14.1 Niacin Clinical Studies
14.2 Niacin Extended-Release Clinical Studies
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Patient Counseling
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Therapy with lipid-altering agents should be only one component of multiple risk factor intervention in individuals at significantly increased risk for atherosclerotic vascular disease due to hyperlipidemia. Niacin therapy is indicated as an adjunct to diet when the response to a diet restricted in saturated fat and cholesterol and other nonpharmacologic measures alone has been inadequate.
- Niacin extended-release tablets are indicated to reduce elevated TC, LDL-C, Apo B and TG levels, and to increase HDL-C in patients with primary hyperlipidemia and mixed dyslipidemia.
- In patients with a history of myocardial infarction and hyperlipidemia, niacin is indicated to reduce the risk of recurrent nonfatal myocardial infarction.
- In patients with a history of coronary artery disease (CAD) and hyperlipidemia, niacin, in combination with a bile acid binding resin, is indicated to slow progression or promote regression of atherosclerotic disease.
- Niacin extended-release tablets in combination with a bile acid binding resin is indicated to reduce elevated TC and LDL-C levels in adult patients with primary hyperlipidemia.
- Niacin is also indicated as adjunctive therapy for treatment of adult patients with severe hypertriglyceridemia who present a risk of pancreatitis and who do not respond adequately to a determined dietary effort to control them.
Limitations of Use
Addition of niacin extended-release tablets did not reduce cardiovascular morbidity or mortality among patients treated with simvastatin in a large, randomized controlled trial (AIM-HIGH) [see Warnings and Precautions (5.1)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Initial Dosing
Niacin extended-release tablets should be taken at bedtime, after a low-fat snack, and doses should be individualized according to patient response. Therapy with niacin extended-release tablets must be initiated at 500 mg at bedtime in order to reduce the incidence and severity of side effects which may occur during early therapy. The recommended dose escalation is shown in Table 1 below.
Table 1. Recommended Dosing
Week(s)
Daily dose
Niacin Extended-Release Tablets Dosage
INITIAL
TITRATION
1 to 4
500 mg
1 Niacin extended-release tablet 500 mg at bedtime
SCHEDULE
5 to 8
1,000 mg
1 Niacin extended-release tablet 1,000 mg or
2 Niacin extended-release tablets 500 mg at bedtime
*
1,500 mg
3 Niacin extended-release tablets 500 mg at bedtime
*
2,000 mg
2 Niacin extended-release tablets 1,000 mg or
4 Niacin extended-release tablets 500 mg at bedtime
* After Week 8, titrate to patient response and tolerance. If response to 1,000 mg daily is inadequate, increase dose to 1,500 mg daily; may subsequently increase dose to 2,000 mg daily. Daily dose should not be increased more than 500 mg in a 4-week period, and doses above 2,000 mg daily are not recommended. Women may respond at lower doses than men.
2.2 Maintenance Dose
The daily dosage of niacin extended-release tablets should not be increased by more than 500 mg in any 4–week period. The recommended maintenance dose is 1,000 mg (two 500 mg tablets or one 1,000 mg tablet) to 2,000 mg (two 1,000 mg tablets or four 500 mg tablets) once daily at bedtime. Doses greater than 2,000 mg daily are not recommended. Women may respond at lower niacin extended-release tablets doses than men [see Clinical Studies (14.2)].
Single-dose bioavailability studies have demonstrated that two of the 500 mg and one of the 1,000 mg tablet strengths are interchangeable but three of the 500 mg and two of the 750 mg tablet strengths are not interchangeable.
Flushing of the skin [see Adverse Reactions (6.1)] may be reduced in frequency or severity by pretreatment with aspirin (up to the recommended dose of 325 mg taken 30 minutes prior to niacin extended-release tablets dose). Tolerance to this flushing develops rapidly over the course of several weeks. Flushing, pruritus, and gastrointestinal distress are also greatly reduced by slowly increasing the dose of niacin and avoiding administration on an empty stomach. Concomitant alcoholic, hot drinks or spicy foods may increase the side effects of flushing and pruritus and should be avoided around the time of niacin extended-release tablets ingestion.
Equivalent doses of niacin extended-release tablets should not be substituted for sustained-release (modified-release, timed-release) niacin preparations or immediate-release (crystalline) niacin [see Warnings and Precautions (5)]. Patients previously receiving other niacin products should be started with the recommended niacin extended-release tablets titration schedule (see Table 1), and the dose should subsequently be individualized based on patient response.
If niacin extended-release tablets therapy is discontinued for an extended period, reinstitution of therapy should include a titration phase (see Table 1).
Niacin extended-release tablets should be taken whole and should not be broken, crushed or chewed before swallowing.
2.3 Dosage in Patients with Renal or Hepatic Impairment
Use of niacin extended-release tablets in patients with renal or hepatic impairment has not been studied. Niacin extended-release tablets are contraindicated in patients with significant or unexplained hepatic dysfunction. Niacin extended-release tablets should be used with caution in patients with renal impairment [see Warnings and Precautions (5)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Niacin extended-release tablets are contraindicated in the following conditions:
- Active liver disease or unexplained persistent elevations in hepatic transaminases [see Warnings and Precautions (5.3)]
- Patients with active peptic ulcer disease
- Patients with arterial bleeding
- Hypersensitivity to niacin or any component of this medication [see Adverse Reactions (6.1)]
-
5 WARNINGS AND PRECAUTIONS
Niacin extended-release preparations should not be substituted for equivalent doses of immediate-release (crystalline) niacin. For patients switching from immediate-release niacin to niacin extended-release, therapy with niacin extended-release should be initiated with low doses (i.e. 500 mg at bedtime) and the niacin extended-release dose should then be titrated to the desired therapeutic response [see Dosage and Administration (2.1)].
Caution should also be used when niacin extended-release is used in patients with unstable angina or in the acute phase of an MI, particularly when such patients are also receiving vasoactive drugs such as nitrates, calcium channel blockers, or adrenergic blocking agents.
Niacin is rapidly metabolized by the liver, and excreted through the kidneys. Niacin extended-release is contraindicated in patients with significant or unexplained hepatic impairment [see Contraindications (4) and Warnings and Precautions (5.3)] and should be used with caution in patients with renal impairment. Patients with a past history of jaundice, hepatobiliary disease, or peptic ulcer should be observed closely during niacin extended-release therapy.
5.1 Mortality and Coronary Heart Disease Morbidity
Niacin extended-release has not been shown to reduce cardiovascular morbidity or mortality among patients already treated with a statin.
The Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides:
Impact on Global Health Outcomes (AIM-HIGH) trial was a randomized placebo-controlled trial of 3,414 patients with stable, previously diagnosed cardiovascular disease. Mean baseline lipid levels were LDL-C 74 mg/dL, HDL-C 35 mg/dL, non-HDL-C 111 mg/dL and median triglyceride level of 163 mg/dL to 177 mg/dL. Ninety-four percent of patients were on background statin therapy prior to entering the trial. All participants received simvastatin, 40 to 80 mg per day, plus ezetimibe 10 mg per day if needed, to maintain an LDL-C level of 40 mg/dL to 80 mg/dL, and were randomized to receive niacin extended-release 1,500 mg/day to 2,000 mg/day (n = 1,718) or matching placebo (IR Niacin, 100 mg to 150 mg, n = 1,696). On-treatment lipid changes at two years for LDL-C were -12.0% for the simvastatin plus niacin extended-release group and -5.5% for the simvastatin plus placebo group. HDL-C increased by 25.0% to 42 mg/dL in the simvastatin plus niacin extended-release group and by 9.8% to 38 mg/dL in the simvastatin plus placebo group (P < 0.001). Triglyceride levels decreased by 28.6% in the simvastatin plus niacin extended-release group and by 8.1% in the simvastatin plus placebo group. The primary outcome was an ITT composite of the first study occurrence of coronary heart disease death, nonfatal myocardial infarction, ischemic stroke, hospitalization for acute coronary syndrome or symptom-driven coronary or cerebral revascularization procedures. The trial was stopped after a mean follow-up period of 3 years owing to a lack of efficacy. The primary outcome occurred in 282 patients in the simvastatin plus niacin extended-release group (16.4%) and in 274 patients in the simvastatin plus placebo group (16.2%) (HR 1.02 [95% CI, 0.87 to 1.21], P = 0.79. In an ITT analysis, there were 42 cases of first occurrence of ischemic stroke reported, 27 (1.6%) in the simvastatin plus niacin extended-release group and 15 (0.9%) in the simvastatin plus placebo group, a non-statistically significant result (HR 1.79, [95%CI = 0.95 to 3.36], p = 0.071). The on-treatment ischemic stroke events were 19 for the simvastatin plus niacin extended-release group and 15 for the simvastatin plus placebo group [see Adverse Reactions (6.1)].
5.2 Skeletal Muscle
Cases of rhabdomyolysis have been associated with concomitant administration of lipid-altering doses (≥ 1 g/day) of niacin and statins. Elderly patients and patients with diabetes, renal failure, or uncontrolled hypothyroidism are particularly at risk. Monitor patients for any signs and symptoms of muscle pain, tenderness, or weakness, particularly during the initial months of therapy and during any periods of upward dosage titration. Periodic serum creatine phosphokinase (CPK) and potassium determinations should be considered in such situations, but there is no assurance that such monitoring will prevent the occurrence of severe myopathy.
5.3 Liver Dysfunction
Cases of severe hepatic toxicity, including fulminant hepatic necrosis, have occurred in patients who have substituted sustained-release (modified-release, timed-release) niacin products for immediate-release (crystalline) niacin at equivalent doses.
Niacin extended-release should be used with caution in patients who consume substantial quantities of alcohol and/or have a past history of liver disease. Active liver diseases or unexplained transaminase elevations are contraindications to the use of niacin extended-release.
Niacin preparations have been associated with abnormal liver tests. In three placebo-controlled clinical trials involving titration to final daily niacin extended-release doses ranging from 500 mg to 3,000 mg, 245 patients received niacin extended-release for a mean duration of 17 weeks. No patient with normal serum transaminase levels (AST, ALT) at baseline experienced elevations to more than 3 times the upper limit of normal (ULN) during treatment with niacin extended-release. In these studies, fewer than 1% (2/245) of niacin extended-release patients discontinued due to transaminase elevations greater than 2 times the ULN.
Liver-related tests should be performed on all patients during therapy with niacin extended-release. Serum transaminase levels, including AST and ALT (SGOT and SGPT), should be monitored before treatment begins, every 6 weeks to 12 weeks for the first year, and periodically thereafter (e.g., at approximately 6-month intervals). Special attention should be paid to patients who develop elevated serum transaminase levels, and in these patients, measurements should be repeated promptly and then performed more frequently. If the transaminase levels show evidence of progression, particularly if they rise to 3 times ULN and are persistent or if they are associated with symptoms of nausea, fever, and/or malaise, the drug should be discontinued.
5.4 Laboratory Abnormalities
Increase in Blood Glucose: Niacin treatment can increase fasting blood glucose. Frequent monitoring of blood glucose should be performed to ascertain that the drug is producing no adverse effects. Diabetic patients may experience a dose-related increase in glucose intolerance. Diabetic or potentially diabetic patients should be observed closely during treatment with niacin extended-release, particularly during the first few months of use or dose adjustment; adjustment of diet and/or hypoglycemic therapy may be necessary.
Reduction in platelet count: Niacin extended-release has been associated with small but statistically significant dose-related reductions in platelet count (mean of -11% with 2,000 mg). Caution should be observed when niacin extended-release is administered concomitantly with anticoagulants; platelet counts should be monitored closely in such patients.
Increase in Prothrombin Time (PT): Niacin extended-release has been associated with small but statistically significant increases in prothrombin time (mean of approximately +4%); accordingly, patients undergoing surgery should be carefully evaluated. Caution should be observed when niacin extended-release is administered concomitantly with anticoagulants; prothrombin time should be monitored closely in such patients.
Increase in Uric Acid: Elevated uric acid levels have occurred with niacin therapy, therefore use with caution in patients predisposed to gout.
Decrease in Phosphorus: In placebo-controlled trials, niacin extended-release has been associated with small but statistically significant, dose-related reductions in phosphorus levels (mean of -13% with 2,000 mg). Although these reductions were transient, phosphorus levels should be monitored periodically in patients at risk for hypophosphatemia.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Mortality and Coronary Heart Disease Morbidity [see Warnings and Precautions (5.1)]
- Skeletal Muscle (rhabdomyolysis) [see Warnings and Precautions (5.2)]
- Liver Dysfunction [see Warnings and Precautions (5.3)]
- Laboratory Abnormalities [see Warnings and Precautions (5.4)]
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
In the placebo-controlled clinical trials database of 402 patients (age range 21 years to 75 years, 33% women, 89% Caucasians, 7% Blacks, 3% Hispanics, 1% Asians) with a median treatment duration of 16 weeks, 16% of patients on niacin extended-release and 4% of patients on placebo discontinued due to adverse reactions. The most common adverse reactions in the group of patients treated with niacin extended-release that led to treatment discontinuation and occurred at a rate greater than placebo were flushing (6% vs. 0%), rash (2% vs. 0%), diarrhea (2% vs. 0%), nausea (1% vs. 0%), and vomiting (1% vs. 0%). The most commonly reported adverse reactions (incidence > 5% and greater than placebo) in the niacin extended-release controlled clinical trial database of 402 patients were flushing, diarrhea, nausea, vomiting, increased cough and pruritus.
In the placebo-controlled clinical trials, flushing episodes (i.e., warmth, redness, itching and/or tingling) were the most common treatment-emergent adverse reactions (reported by as many as 88% of patients) for niacin extended-release. Spontaneous reports suggest that flushing may also be accompanied by symptoms of dizziness, tachycardia, palpitations, shortness of breath, sweating, burning sensation/skin burning sensation, chills, and/or edema, which in rare cases may lead to syncope. In pivotal studies, 6% (14/245) of niacin extended-release patients discontinued due to flushing. In comparisons of immediate-release (IR) niacin and niacin extended-release tablets, although the proportion of patients who flushed was similar, fewer flushing episodes were reported by patients who received niacin extended-release. Following 4 weeks of maintenance therapy at daily doses of 1,500 mg, the incidence of flushing over the 4-week period averaged 8.6 events per patient for IR niacin versus 1.9 following niacin extended-release.
Other adverse reactions occurring in ≥ 5% of patients treated with niacin extended-release and at an incidence greater than placebo are shown in Table 2 below.
Table 2. Treatment-Emergent Adverse Reactions by Dose Level in ≥ 5% of Patients and at an Incidence Greater than Placebo; Regardless of Causality Assessment in Placebo-Controlled Clinical Trials
Placebo-Controlled Studies Niacin Extended-release Treatment@
Recommended Daily Maintenance Doses †
Placebo
(n = 157)
500 mg‡
(n = 87)
1,000 mg
(n = 110)
1,500 mg
(n = 136)
2,000 mg
(n = 95)
%
%
%
%
%
Gastrointestinal Disorders
Diarrhea
13
7
10
10
14
Nausea
7
5
6
4
11
Vomiting
4
0
2
4
9
Respiratory
Cough, Increased
6
3
2
< 2
8
Skin and Subcutaneous Tissue Disorders
Pruritus
2
8
0
3
0
Rash
0
5
5
5
0
Vascular Disorders
Flushing&
19
68
69
63
55
Note: Percentages are calculated from the total number of patients in each column.
† Adverse reactions are reported at the initial dose where they occur.
@ Pooled results from placebo-controlled studies; for niacin extended-release, n = 245 and median treatment duration = 16 weeks. Number of niacin patients (n) are not additive across doses.
‡ The 500 mg/day dose is outside the recommended daily maintenance dosing range [see Dosage and Administration (2.2)].
& 10 patients discontinued before receiving 500 mg, therefore they were not included.
In general, the incidence of adverse events was higher in women compared to men.
Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides:
Impact on Global Health Outcomes (AIM-HIGH)
In AIM-HIGH involving 3,414 patients (mean age of 64 years, 15% women, 92% Caucasians, 34% with diabetes mellitus) with stable, previously diagnosed cardiovascular disease, all patients received simvastatin, 40 to 80 mg per day, plus ezetimibe 10 mg per day if needed, to maintain an LDL-C level of 40 mg/dL to 80 mg/dL, and were randomized to receive niacin extended-release 1,500 mg/day to 2,000 mg/day (n=1,718) or matching placebo (IR Niacin, 100 mg to 150 mg, n=1,696). The incidence of the adverse reactions of “blood glucose increased” (6.4% vs. 4.5%) and “diabetes mellitus” (3.6% vs. 2.2%) was significantly higher in the simvastatin plus niacin extended-release group as compared to the simvastatin plus placebo group. There were 5 cases of rhabdomyolysis reported, 4 (0.2%) in the simvastatin plus niacin extended-release group and one (<0.1%) in the simvastatin plus placebo group.
6.2 Post-marketing Experience
The following additional adverse reactions have been identified during post-approval use of niacin extended-release. Because the below reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac disorders: tachycardia, palpitations, atrial fibrillation, other cardiac arrhythmias
Eye disorders: blurred vision, macular edema
Gastrointestinal disorders: peptic ulcers, eructation, flatulence
Hepatobiliary disorders: hepatitis, jaundice
Immune system disorders: hypersensitivity reactions (including anaphylaxis, angioedema, urticaria, flushing, dyspnea, tongue edema, larynx edema, face edema, peripheral edema, laryngismus and vesiculobullous rash)
Metabolism and nutrition disorders: decreased glucose tolerance, gout
Musculoskeletal and connective tissue disorders: myalgia, myopathy
Nervous system disorders: dizziness, insomnia, asthenia, nervousness, paresthesia, migraine
Respiratory, thoracic and mediastinal disorders: dyspnea
Skin and subcutaneous tissue disorders: maculopapular rash, dry skin, sweating, burning sensation/skin burning sensation, skin discoloration, acanthosis nigricans
Vascular disorders: syncope, hypotension, postural hypotension
Clinical Laboratory Abnormalities
Chemistry: Elevations in serum transaminases, LDH, fasting glucose, uric acid, total bilirubin, amylase and creatine kinase, and reduction in phosphorus.
Hematology: Slight reductions in platelet counts and prolongation in prothrombin time.
-
7 DRUG INTERACTIONS
7.1 Statins
Caution should be used when prescribing niacin (≥ 1 gm/day) with statins as these drugs can increase risk of myopathy/rhabdomyolysis [see Warnings and Precautions (5) and Clinical Pharmacology (12.3)].
7.2 Bile Acid Sequestrants
An in vitro study results suggest that the bile acid-binding resins have high niacin binding capacity. Therefore, 4 to 6 hours, or as great an interval as possible, should elapse between the ingestion of bile acid-binding resins and the administration of niacin extended-release [see Clinical Pharmacology (12.3)].
7.3 Aspirin
Concomitant aspirin may decrease the metabolic clearance of nicotinic acid. The clinical relevance of this finding is unclear.
7.4 Antihypertensive Therapy
Niacin may potentiate the effects of ganglionic blocking agents and vasoactive drugs resulting in postural hypotension.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Discontinue niacin extended-release when pregnancy is recognized in patients receiving the drug for the treatment of hyperlipidemia. Assess the individual risks and benefits of continuing niacin extended-release during pregnancy in patients receiving the drug for the treatment of hypertriglyceridemia. Advise patients to inform their healthcare provider of a known or suspected pregnancy.
The potential for embryofetal toxicity with the doses of niacin in niacin extended-release tablets is unknown. The available data on niacin extended-release use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted with niacin or with niacin extended-release tablets. Treatment of hypercholesterolemia is not generally necessary during pregnancy. Atherosclerosis is a chronic process and the discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of long-term therapy of primary hypercholesterolemia for most patients.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
Niacin is present in human milk and the amount of niacin increases with maternal supplementation. There is no information on the effects of the doses of niacin in niacin extended-release tablets on the breastfed infant or the effects on milk production. Because of the potential for serious adverse reactions in breastfeeding infants, including hepatotoxicity, advise patients not to breastfeed during treatment with niacin extended-release.
8.4 Pediatric Use
Safety and effectiveness of niacin therapy in pediatric patients (≤ 16 years) have not been established.
8.5 Geriatric Use
Of 979 patients in clinical studies of niacin extended-release, 21% of the patients were age 65 and over. No overall differences in safety and effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
No studies have been performed in this population. Niacin extended-release should be used with caution in patients with renal impairment [see Warnings and Precautions (5)].
8.7 Hepatic Impairment
No studies have been performed in this population. Niacin extended-release should be used with caution in patients with a past history of liver disease and/or who consume substantial quantities of alcohol. Active liver disease, unexplained transaminase elevations and significant or unexplained hepatic dysfunction are contraindications to the use of niacin extended-release [see Contraindications (4) and Warnings and Precautions (5.3)].
- 10 OVERDOSAGE
-
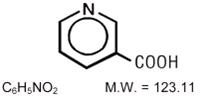
11 DESCRIPTION
Niacin extended-release tablets contain niacin, USP, which at therapeutic doses is an antihyperlipidemic agent. Niacin, USP (nicotinic acid, or 3-pyridinecarboxylic acid) is a white, crystalline powder, very soluble in water, with the following structural formula:
Niacin extended-release tablets are unscored, light orange to orange, film-coated tablets for oral administration and are available in two tablet strengths containing 500 mg and 1,000 mg niacin. Niacin extended-release tablets also contain the following inactive ingredients: FD&C yellow #6/sunset yellow FCF aluminum lake, hydroxyethyl cellulose, hypromellose, iron oxide red, iron oxide yellow, polyethylene glycol 400, stearic acid and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism by which niacin alters lipid profiles has not been well defined. It may involve several actions including partial inhibition of release of free fatty acids from adipose tissue, and increased lipoprotein lipase activity, which may increase the rate of chylomicron triglyceride removal from plasma. Niacin decreases the rate of hepatic synthesis of VLDL and LDL, and does not appear to affect fecal excretion of fats, sterols, or bile acids.
12.3 Pharmacokinetics
Absorption
Due to extensive and saturable first-pass metabolism, niacin concentrations in the general circulation are dose dependent and highly variable. Time to reach the maximum niacin plasma concentrations was about 5 hours following niacin extended-release. To reduce the risk of gastrointestinal (GI) upset, administration of niacin extended-release with a low-fat meal or snack is recommended.
Single-dose bioavailability studies have demonstrated that the 500 mg and 1,000 mg tablet strengths are dosage form equivalent but the 500 mg and 750 mg tablet strengths are not dosage form equivalent.
Metabolism
The pharmacokinetic profile of niacin is complicated due to extensive first-pass metabolism that is dose-rate specific and, at the doses used to treat dyslipidemia, saturable. In humans, one pathway is through a simple conjugation step with glycine to form nicotinuric acid (NUA). NUA is then excreted in the urine, although there may be a small amount of reversible metabolism back to niacin. The other pathway results in the formation of nicotinamide adenine dinucleotide (NAD). It is unclear whether nicotinamide is formed as a precursor to, or following the synthesis of, NAD. Nicotinamide is further metabolized to at least N-methylnicotinamide (MNA) and nicotinamide-N-oxide (NNO). MNA is further metabolized to two other compounds, N-methyl-2-pyridone-5-carboxamide (2PY) and N-methyl-4-pyridone-5-carboxamide (4PY). The formation of 2PY appears to predominate over 4PY in humans. At the doses used to treat hyperlipidemia, these metabolic pathways are saturable, which explains the nonlinear relationship between niacin dose and plasma concentrations following multiple-dose niacin extended-release administration.
Nicotinamide does not have hypolipidemic activity; the activity of the other metabolites is unknown.
Elimination
Following single and multiple doses, approximately 60% to 76% of the niacin dose administered as niacin extended-release was recovered in urine as niacin and metabolites; up to 12% was recovered as unchanged niacin after multiple dosing. The ratio of metabolites recovered in the urine was dependent on the dose administered.
Pediatric Use
No pharmacokinetic studies have been performed in this population (≤ 16 years) [see Use in Specific Populations (8.4)].
Geriatric Use
No pharmacokinetic studies have been performed in this population (> 65 years) [see Use in Specific Populations (8.5)].
Renal Impairment
No pharmacokinetic studies have been performed in this population. Niacin extended-release should be used with caution in patients with renal disease [see Warnings and Precautions (5)].
Hepatic Impairment
No pharmacokinetic studies have been performed in this population. Active liver disease, unexplained transaminase elevations and significant or unexplained hepatic dysfunction are contraindications to the use of niacin extended-release [see Contraindications (4) and Warnings and Precautions (5.3)].
Gender
Steady-state plasma concentrations of niacin and metabolites after administration of niacin extended-release tablets are generally higher in women than in men, with the magnitude of the difference varying with dose and metabolite. This gender differences observed in plasma levels of niacin and its metabolites may be due to gender-specific differences in metabolic rate or volume of distribution. Recovery of niacin and metabolites in urine, however, is generally similar for men and women, indicating that absorption is similar for both genders [see Gender (8.8)].
Drug interactions
Fluvastatin
Niacin did not affect fluvastatin pharmacokinetics [see Drug Interactions (7.1)].
Lovastatin
When niacin extended-release 2,000 mg and lovastatin 40 mg were co-administered, niacin extended-release increased lovastatin Cmax and AUC by 2% and 14%, respectively, and decreased lovastatin acid Cmax and AUC by 22% and 2%, respectively. Lovastatin reduced niacin extended-release bioavailability by 2% to 3% [see Drug Interactions (7.1)].
Simvastatin
When niacin extended-release 2,000 mg and simvastatin 40 mg were co-administered, niacin extended-release increased simvastatin Cmax and AUC by 1% and 9%, respectively, and simvastatin acid Cmax and AUC by 2% and 18%, respectively. Simvastatin reduced niacin extended-release bioavailability by 2% [see Drug Interactions (7.1)].
Bile Acid Sequestrants
An in vitro study was carried out investigating the niacin-binding capacity of colestipol and cholestyramine. About 98% of available niacin was bound to colestipol, with 10% to 30% binding to cholestyramine [see Drug Interactions (7.2)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis and Mutagenesis and Impairment of Fertility
Niacin administered to mice for a lifetime as a 1% solution in drinking water was not carcinogenic. The mice in this study received approximately 6 to 8 times a human dose of 3,000 mg/day as determined on a mg/m2 basis. Niacin was negative for mutagenicity in the Ames test. No studies on impairment of fertility have been performed. No studies have been conducted with niacin extended-release regarding carcinogenesis, mutagenesis, or impairment of fertility.
-
14 CLINICAL STUDIES
14.1 Niacin Clinical Studies
Niacin’s ability to reduce mortality and the risk of definite, nonfatal myocardial infarction (MI) has been assessed in long-term studies. The Coronary Drug Project, completed in 1975, was designed to assess the safety and efficacy of niacin and other lipid-altering drugs in men 30 to 64 years old with a history of MI. Over an observation period of 5 years, niacin treatment was associated with a statistically significant reduction in nonfatal, recurrent MI. The incidence of definite, nonfatal MI was 8.9% for the 1,119 patients randomized to nicotinic acid versus 12.2% for the 2,789 patients who received placebo (p < 0.004). Total mortality was similar in the two groups at 5 years (24.4% with nicotinic acid versus 25.4% with placebo; p=N.S.). At the time of a 15-year follow-up, there were 11% (69) fewer deaths in the niacin group compared to the placebo cohort (52.0% versus 58.2%; p=0.0004). However, mortality at 15 years was not an original endpoint of the Coronary Drug Project. In addition, patients had not received niacin for approximately 9 years, and confounding variables such as concomitant medication use and medical or surgical treatments were not controlled.
The Cholesterol-Lowering Atherosclerosis Study (CLAS) was a randomized, placebo-controlled, angiographic trial testing combined colestipol and niacin therapy in 162 non-smoking males with previous coronary bypass surgery. The primary, per-subject cardiac endpoint was global coronary artery change score. After 2 years, 61% of patients in the placebo cohort showed disease progression by global change score (n=82), compared with only 38.8% of drug-treated subjects (n=80), when both native arteries and grafts were considered (p < 0.005); disease regression also occurred more frequently in the drug-treated group (16.2% versus 2.4%; p = 0.002). In a follow-up to this trial in a subgroup of 103 patients treated for 4 years, again, significantly fewer patients in the drug-treated group demonstrated progression than in the placebo cohort (48% versus 85%, respectively; p < 0.0001).
The Familial Atherosclerosis Treatment Study (FATS) in 146 men ages 62 and younger with Apo B levels ≥125 mg/dL, established coronary artery disease, and family histories of vascular disease, assessed change in severity of disease in the proximal coronary arteries by quantitative arteriography. Patients were given dietary counseling and randomized to treatment with either conventional therapy with double placebo (or placebo plus colestipol if the LDL-C was elevated); lovastatin plus colestipol; or niacin plus colestipol. In the conventional therapy group, 46% of patients had disease progression (and no regression) in at least one of nine proximal coronary segments; regression was the only change in 11%. In contrast, progression (as the only change) was seen in only 25% in the niacin plus colestipol group, while regression was observed in 39%. Though not an original endpoint of the trial, clinical events (death, MI, or revascularization for worsening angina) occurred in 10 of 52 patients who received conventional therapy, compared with 2 of 48 who received niacin plus colestipol.
14.2 Niacin Extended-Release Clinical Studies
Placebo-Controlled Clinical Studies in Patients with Primary Hyperlipidemia and Mixed Dyslipidemia: In two randomized, double-blind, parallel, multi-center, placebo-controlled trials, niacin extended-release dosed at 1,000 mg, 1,500 mg or 2,000 mg daily at bedtime with a low-fat snack for 16 weeks (including 4 weeks of dose escalation) favorably altered lipid profiles compared to placebo (Table 3). Women appeared to have a greater response than men at each niacin extended-release dose level (see Gender Effect, below).
Table 3. Lipid Response to Niacin Extended-Release Therapy
Mean Percent Change from Baseline to Week 16*
Treatment
n
TC
LDL-C
HDL-C
TG
Apo B
Niacin Extended-Release 1,000 mg at
41
-3
-5
+18
-21
-6
bedtime
Niacin Extended-Release 2,000 mg at
41
-10
-14
+22
-28
-16
bedtime
Placebo
40
0
-1
+4
0
+1
Niacin Extended-Release 1,500 mg at
76
-8
-12
+20
-13
-12
bedtime
Placebo
73
+2
+1
+2
+12
+1
n = number of patients at baseline;
* Mean percent change from baseline for all niacin extended-release doses was significantly different (p < 0.05) from placebo.
In a double-blind, multi-center, forced dose-escalation study, monthly 500 mg increases in niacin extended-release dose resulted in incremental reductions of approximately 5% in LDL-C and Apo B levels in the daily dose range of 500 mg through 2,000 mg (Table 4). Women again tended to have a greater response to niacin extended-release than men (see Gender Effect, below).
Table 4. Lipid Response in Dose-Escalation Study
Mean Percent Change from Baseline*
Treatment
n
TC
LDL- C
HDL-C
TG
Apo B
Placebo‡
44
-2
-1
+5
-6
-2
Niacin extended-release
87
500 mg at bedtime
-2
-3
+10
-5
-2
1,000 mg at bedtime
-5
-9
+15
-11
-7
1,500 mg at bedtime
-11
-14
+22
-28
-15
2,000 mg at bedtime
-12
-17
+26
-35
-16
n = number of patients enrolled;
‡ Placebo data shown are after 24 weeks of placebo treatment.
* For all niacin extended-release doses except 500 mg, mean percent change from baseline was significantly different (p < 0.05) from placebo for all lipid parameters shown.
Pooled results for major lipids from these three placebo-controlled studies are shown below (Table 5).
Table 5. Selected Lipid Response to Niacin Extended-Release in Placebo-Controlled Clinical Studies*
Mean Baseline and Median Percent Change from Baseline
(25th, 75th Percentiles)
Niacin Extended-ReleaseDose
n
LDL-C
HDL-C
TG
1,000 mg at bedtime
104
Baseline (mg/dL)
218
45
172
Percent Change
-7 (-15, 0)
+14 (+7, +23)
-16 (-34, +3)
1500 mg at bedtime
120
Baseline (mg/dL)
212
46
171
Percent Change
-13 (-21, -4)
+19 (+9, +31)
-25 (-45, -2)
2000 mg at bedtime
85
Baseline (mg/dL)
220
44
160
Percent Change
-16 (-26, -7)
+22 (+15, +34)
-38 (-52, -14)
* Represents pooled analyses of results; minimum duration on therapy at each dose was 4 weeks.
Gender Effect: Combined data from the three placebo-controlled niacin extended-release studies in patients with primary hyperlipidemia and mixed dyslipidemia suggest that, at each niacin extended-release dose level studied, changes in lipid concentrations are greater for women than for men (Table 6).
Table 6. Effect of Gender on Niacin Extended-Release Dose Response
Mean Percent Change from Baseline
Niacin Extended-Release
n LDL-C
HDL-C
TG
Apo B
Dose
(M/F)
M
F
M
F
M
F
M
F
500 mg at bedtime
50/37
-2
-5
+11
+8
-3
-9
-1
-5
1,000 mg at bedtime
76/52
-6*
-11*
+14
+20
-10
-20
-5*
-10*
1,500 mg at bedtime
104/59
-12
-16
+19
+24
-17
-28
-13
-15
2,000 mg at bedtime
75/53
-15
-18
+23
+26
-30
-36
-16
-16
n = number of male/female patients enrolled.
* Percent change significantly different between genders (p < 0.05).
Other Patient Populations: In a double-blind, multi-center, 19-week study the lipid-altering effects of niacin extended-release (forced titration to 2,000 mg at bedtime) were compared to baseline in patients whose primary lipid abnormality was a low level of HDL-C (HDL-C ≤ 40 mg/dL, TG ≤ 400 mg/dL, and LDL-C ≤ 160, or <130 mg/dL in the presence of CHD). Results are shown below (Table 7).
Table 7. Lipid Response to Niacin Extended-Release in Patients with Low HDL-C
Mean Baseline and Mean Percent Change from Baseline*
n
TC
LDL-C
HDL-C
TG
Apo B†
Baseline
88
190
120
31
194
106
(mg/dL)
Week 19
71
-3
0
+26
-30
-9
(% Change)
n = number of patients
* Mean percent change from baseline was significantly different (p < 0.05) for all lipid parameters shown except LDL-C.
† n = 72 at baseline and 69 at week 19.
At niacin extended-release 2,000 mg/day, median changes from baseline (25th, 75th percentiles) for LDL-C, HDL-C, and TG were -3% (-14, +12%), +27% (+13, +38%), and -33% (-50, -19%), respectively.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Niacin extended-release tablets, 500 mg are supplied as light orange to orange colored, round shaped, film-coated tablets debossed with “AN 321” on one side and plain on the other side.
They are available as follows:
Bottles of 30: NDC 65162-321-03
Bottles of 90: NDC 65162-321-09
Bottles of 500: NDC 65162-321-50
Niacin extended-release tablets, 1000 mg are supplied as light orange to orange colored, capsule shaped, film-coated tablets debossed with “AN 323” on one side and plain on the other side.
They are available as follows:
Bottles of 30: NDC 65162-323-03
Bottles of 90: NDC 65162-323-09
Bottles of 500: NDC 65162-323-50
Storage: Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
17.1 Patient Counseling
Patients should be advised to adhere to their National Cholesterol Education Program (NCEP) recommended diet, a regular exercise program, and periodic testing of a fasting lipid panel.
Patients should be advised to inform other healthcare professionals prescribing a new medication that they are taking niacin extended-release.
The patient should be informed of the following:
Dosing Time
Niacin extended-release tablets should be taken at bedtime, after a low-fat snack. Administration on an empty stomach is not recommended.
Tablet Integrity
Niacin extended-release tablets should not be broken, crushed or chewed, but should be swallowed whole.
Dosing Interruption
If dosing is interrupted for any length of time, their physician should be contacted prior to restarting therapy; re-titration is recommended.
Muscle Pain
Notify their physician of any unexplained muscle pain, tenderness, or weakness promptly. They should discuss all medication, both prescription and over the counter, with their physician.
Flushing
Flushing (warmth, redness, itching and/or tingling of the skin) is a common side effect of niacin therapy that may subside after several weeks of consistent niacin extended-release use. Flushing may vary in severity and is more likely to occur with initiation of therapy, or during dose increases. By dosing at bedtime, flushing will most likely occur during sleep. However, if awakened by flushing at night, the patient should get up slowly, especially if feeling dizzy, feeling faint, or taking blood pressure medications. Advise patients of the symptoms of flushing and how they differ from the symptoms of a myocardial infarction.
Use of Aspirin Medication
Taking aspirin (up to the recommended dose of 325 mg) approximately 30 minutes before dosing can minimize flushing.
Diet
Avoid ingestion of alcohol, hot beverages and spicy foods around the time of taking niacin extended-release to minimize flushing.
Supplements
Notify their physician if they are taking vitamins or other nutritional supplements containing niacin or nicotinamide.
Dizziness
Notify their physician if symptoms of dizziness occur.
Diabetics
If diabetic, to notify their physician of changes in blood glucose.
Pregnancy
Advise patients to inform their healthcare provider of a known or suspected pregnancy to discuss if niacin extended-release should be discontinued [see Use in Specific Populations (8.1)].
Lactation
Advise patients not to breastfeed during treatment with niacin extended-release tablet.
Manufactured By:
Amneal Pharmaceuticals Pvt. Ltd.
Ahmedabad 382220, INDIADistributed By:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807Rev. 09-2023-05
-
PATIENT INFORMATION
Niacin (nye’ a sin)
Extended-Release Tablets
for oral useRead this information carefully before you start taking niacin extended-release tablet(s) and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment.
What are niacin extended-release tablet(s)?
Niacin extended-release tablet(s) are a prescription medicine used with diet and exercise to increase the good cholesterol (HDL) and lower the bad cholesterol (LDL) and fats (triglycerides) in your blood.
- Niacin extended-release tablet(s) is also used to lower the risk of heart attack in people who have had a heart attack and have high cholesterol.
- In people with coronary artery disease and high cholesterol, niacin extended-release tablet(s), when used with a bile acid-binding resin (another cholesterol medicine) can slow down or lessen the build-up of plaque (fatty deposits) in your arteries.
- In people with heart problems and well-controlled cholesterol, taking niacin extended-release tablet(s) with another cholesterol-lowering medicine (simvastatin) does not reduce heart attacks or strokes more than taking simvastatin alone.
Who should not take niacin extended-release tablet(s)?
Do not take niacin extended-release tablet(s) if you have:
- liver problems.
- a stomach ulcer.
- bleeding problems.
- an allergy to niacin or any of the ingredients in niacin extended-release tablet(s). See the end of this Patient Information leaflet for a complete list of ingredients in niacin extended-release tablet(s).
What should I tell my doctor before taking niacin extended-release tablet(s)?
Before you take niacin extended-release tablet(s), tell your doctor about all your medical problems including, if you:
- have diabetes. Tell your doctor if your blood sugar levels change after you take niacin extended-release tablet(s).
- have gout.
- have kidney problems.
- are pregnant or plan to become pregnant. It is not known if niacin extended-release tablet(s) will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant while taking niacin extended-release tablet(s).
- are breastfeeding or plan to breastfeed. Niacin can pass into your breast milk. You and your doctor should decide if you will take niacin extended-release tablet(s) or breastfeed. You should not do both. Talk to your doctor about the best way to feed your baby if you take niacin extended-release tablet(s).
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, herbal supplements or other nutritional supplements containing niacin or nicotinamide. Niacin extended-release tablet(s) and other medicines may affect each other causing side effects. Niacin extended-release tablet(s) may affect the way other medicines work, and other medicines may affect how niacin extended-release tablet(s) works.
Especially tell your doctor if you take:
- other medicines to lower cholesterol or triglycerides
- aspirin
- blood pressure medicines
- blood thinner medicines
- large amounts of alcohol
How should I take niacin extended-release tablet(s)?
- Take niacin extended-release tablet(s) exactly as your doctor tells you to take it.
- Take niacin extended-release tablet(s) whole. Do not break, crush or chew niacin extended-release tablet(s) before swallowing.
- Take niacin extended-release tablet(s) 1 time a day at bedtime after a low-fat snack. Niacin extended-release tablet(s) should not be taken on an empty stomach.
- All forms of niacin are not the same as niacin extended-release tablet(s). Do not switch between forms of niacin without first talking to your doctor as severe liver damage can occur.
- Do not change your dose or stop taking niacin extended-release tablet(s) unless your doctor tells you to.
- If you need to stop taking niacin extended-release tablet(s), call your doctor before you start taking niacin extended-release tablet(s) again. Your doctor may need to lower your dose of niacin extended-release tablet(s).
- If you take too much niacin extended-release tablet(s), call your doctor right away.
- Medicines used to lower your cholesterol called bile acid resins, such as colestipol and cholestyramine, should not be taken at the same time of day as niacin extended-release tablet(s). You should take niacin extended-release tablet(s) and the bile acid resin medicine at least 4 to 6 hours apart.
- Your doctor may do blood tests before you start taking niacin extended-release tablet(s) and during your treatment. You should see your doctor regularly to check your cholesterol and triglyceride levels and to check for side effects.
What are the possible side effects of niacin extended-release tablet(s)?
Niacin extended-release tablet(s) may cause serious side effects, including:
- unexplained muscle pain, tenderness or weakness
- severe liver problems. Signs of liver problems include:
- increased tiredness
- dark colored urine (tea-colored)
- loss of appetite
- light colored stools
- nausea
- right upper stomach (abdomen) pain
- yellowing of your skin or whites of your eye
- itchy skin
- high blood sugar level (glucose)
Call your doctor right away if you have any of the side effects listed above.
The most common side effects of niacin extended-release tablet(s) include:
- flushing
- diarrhea
- nausea
- vomiting
- increased cough
- rash
- itching
Flushing is the most common side effect of niacin extended-release tablet(s). Flushing happens when tiny blood vessels near the surface of the skin (especially on the face, neck, chest and/or back) open wider. Symptoms of flushing may include any or all of the following:
- warmth
- redness
- itching
- tingling of the skin
Flushing does not always happen. If it does, it is usually within 2 to 4 hours after taking a dose of niacin extended-release tablet(s). Flushing may last for a few hours. Flushing is more likely to happen when you first start taking niacin extended-release tablet(s) or when your dose of niacin extended-release tablet(s) is increased. Flushing may get better after several weeks.
If you wake up at night because of flushing, get up slowly, especially if you:
- feel dizzy or faint
- take blood pressure medicines
To lower your chance of flushing:
- Ask your doctor if you can take aspirin to help lower the flushing side effect from niacin extended-release tablets. You can take aspirin (up to the recommended dose of 325 mg) about 30 minutes before you take niacin extended-release tablets to help lower the flushing side effect.
- Do not drink hot beverages (including coffee), alcohol, or eat spicy foods around the time you take niacin extended-release tablet(s).
- Take niacin extended-release tablet(s) with a low-fat snack to lessen upset stomach.
People with high cholesterol and heart disease are at risk for a heart attack. Symptoms of a heart attack may be different from a flushing reaction from niacin extended-release tablet(s). The following may be symptoms of a heart attack due to heart disease and not a flushing reaction:
- chest pain
- pain in other areas of your upper body such as one or both arms, back, neck, jaw or stomach
- shortness of breath
- sweating
- nausea
- lightheadedness
The chest pain you have with a heart attack may feel like uncomfortable pressure, squeezing, fullness or pain that lasts more than a few minutes, or that goes away and comes back. Heart attacks may be sudden and intense, but often start slowly, with mild pain or discomfort.
Call your doctor right away if you have any symptoms of a heart attack.
Tell your doctor if you have any side effect that bothers you or does not go away.
These are not all the possible side effects of niacin extended-release tablet(s). For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store niacin extended-release tablet(s)?
- Store niacin extended-release tablets at 20° to 25°C (68° to 77°F).
General information about the safe and effective use of niacin extended-release tablet(s).
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use niacin extended-release tablet(s) for a condition for which it was not prescribed. Do not give niacin extended-release tablet(s) to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about niacin extended-release tablet(s). If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about niacin extended-release tablet(s) that is written for health professionals.
What are the ingredients in niacin extended-release tablet(s)?
Active ingredient: Niacin, USP
Inactive Ingredients: FD&C yellow #6/sunset yellow FCF aluminum lake, hydroxyethyl cellulose, hypromellose, iron oxide red, iron oxide yellow, polyethylene glycol 400, stearic acid and titanium dioxide
For more information, go to www.amneal.com or call Amneal Pharmaceuticals at 1-877-835-5472.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured By:
Amneal Pharmaceuticals Pvt. Ltd.
Ahmedabad 382220, INDIADistributed By:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Rev. 09-2023-05
Dispense with Patient Information available at:
documents.amneal.com/mg/ppi-niacin-er-tab.pdf - PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NIACIN
niacin tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65162-321 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIACIN (UNII: 2679MF687A) (NIACIN - UNII:2679MF687A) NIACIN 500 mg Inactive Ingredients Ingredient Name Strength FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYDROXYETHYL CELLULOSE (4000 MPA.S AT 1%) (UNII: ZYD53NBL45) HYPROMELLOSES (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color orange Score no score Shape ROUND Size 11mm Flavor Imprint Code AN;321 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65162-321-03 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/27/2015 2 NDC:65162-321-09 90 in 1 BOTTLE; Type 0: Not a Combination Product 07/27/2015 3 NDC:65162-321-50 500 in 1 BOTTLE; Type 0: Not a Combination Product 07/27/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203578 07/27/2015 NIACIN
niacin tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65162-323 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIACIN (UNII: 2679MF687A) (NIACIN - UNII:2679MF687A) NIACIN 1000 mg Inactive Ingredients Ingredient Name Strength FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYDROXYETHYL CELLULOSE (4000 MPA.S AT 1%) (UNII: ZYD53NBL45) HYPROMELLOSES (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color orange Score no score Shape CAPSULE Size 19mm Flavor Imprint Code AN;323 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65162-323-03 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/27/2015 2 NDC:65162-323-09 90 in 1 BOTTLE; Type 0: Not a Combination Product 07/27/2015 3 NDC:65162-323-50 500 in 1 BOTTLE; Type 0: Not a Combination Product 07/27/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203578 07/27/2015 Labeler - Amneal Pharmaceuticals LLC (123797875)