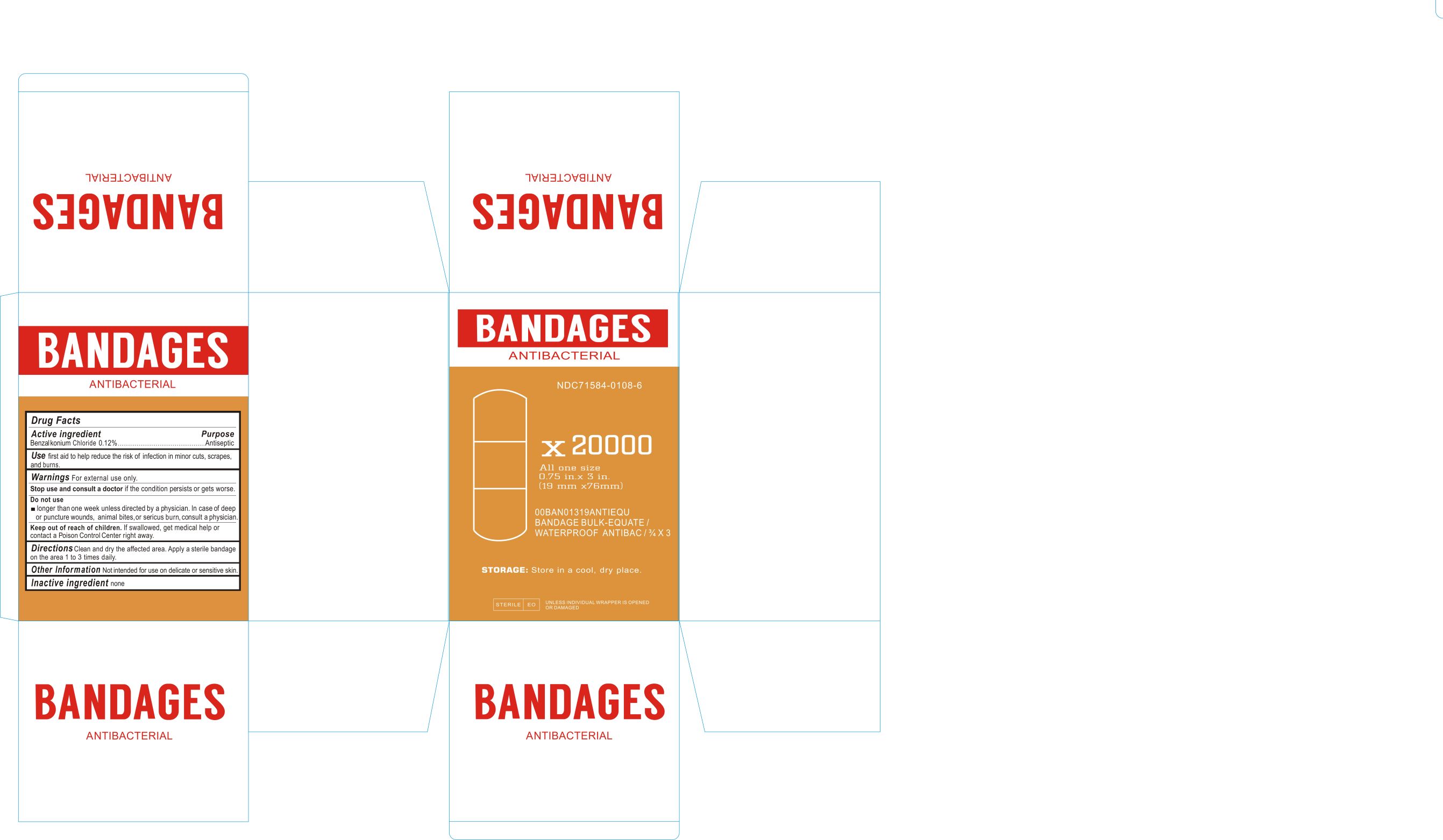
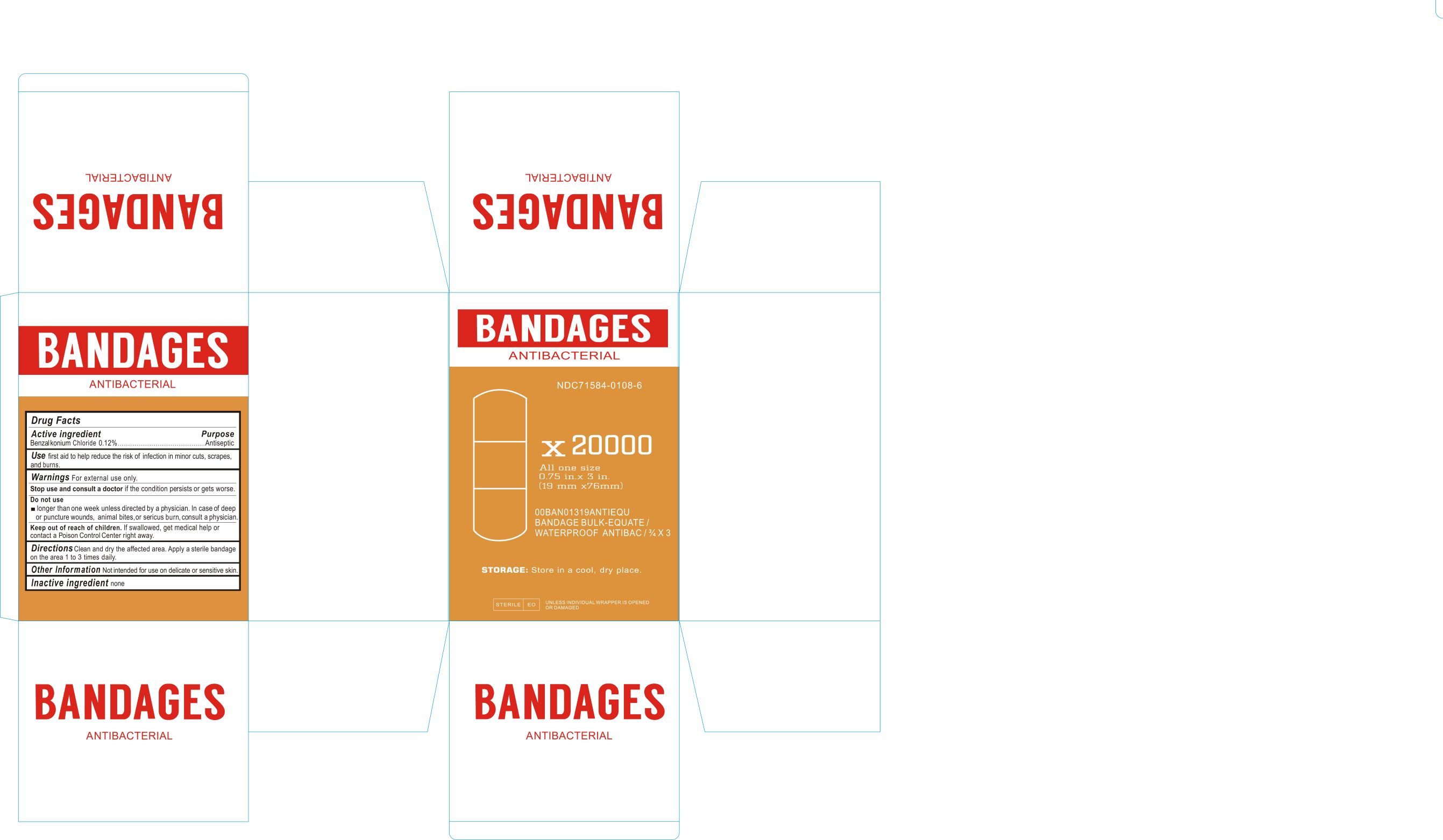
Label: ANTIBACTERIAL BANDAGES- benzalkonium chloride dressing
- NDC Code(s): 71584-0108-1, 71584-0108-2, 71584-0108-3, 71584-0108-4, view more
- Packager: Guangdong Comfort Medical Products Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTSBenzalkonium Chloride 0.12%
-
PurposeAntiseptic
-
USEFirst aid to help reduce the risk of infection in minor cuts, scrapes, and burns.
-
WARNINGSFor external use only. Stop use and consult a doctor if the condition persists or gets worse. Do not use longer than 1 week unless directed by a physician. In case of deep or puncture wounds ...
-
DIRECTIONSClean and dry the affected area. Apply a sterile bandage on the area 1 to 3 times daily.
-
Other InformationNot intended for use on delicate or sensitive skin.
-
INACTIVE INGREDIENTNone
-
Keep out of reach of childrenKeep out of reach of children. if swallowed get medical help or contact Poison control Center right away.
-
Dosage and administrationApply a sterile bandage on the affected area 1 to 3 times daily.
-
Label principal display panelNDC 71584-0108-1 - ANTIBACTERIAL BANDAGES - Drug Facts - ACTIVE INGREDIENTS - Benzalkonium Chloride 0.12% Purpose - Antiseptic - USE - First aid to help reduce the risk of infection in ...
-
INGREDIENTS AND APPEARANCEProduct Information