Label: ANEW PLATINUM DAY- octinoxate, octisalate, oxybenzone, avobenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 71110-0009-1 - Packager: Avon Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 1, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
• apply liberally 15 minutes before sun exposure
• children under 6 months of age: ask a doctor
• reapply at least every 2 hours
• use a water resistant sunscreen if swimming or sweating
• Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. – 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses - STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients: WATER, GLYCERIN, SILICA, DIMETHICONE, STEARIC ACID, STEARYL ALCOHOL, NONENOL, SAPINDUS RARAK FRUIT EXTRACT, POUZOLZIA PENTANDRA EXTRACT, METHOXYBENZOYL METHYLSULFONYL OXOPIPERIDINYL PIPERAZINECARBOXAMIDE, THIAZOLYLALANINE, SACCHAROMYCES/PLATINUM FERMENT, TRISILOXANE, DIMETHICONOL, MYRISTYL MYRISTATE, MALTODEXTRIN, PEG-100 STEARATE, CAPRYLYL GLYCOL,GLYCERYL STEARATE, SODIUM POLYACRYLATE, POLYURETHANE-40, POTASSIUM HYDROXIDE, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, DISODIUM EDTA, 1,2-HEXANEDIOL, PARFUM.
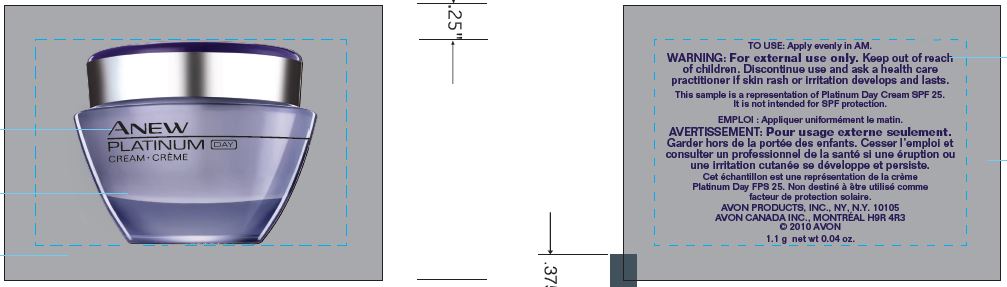
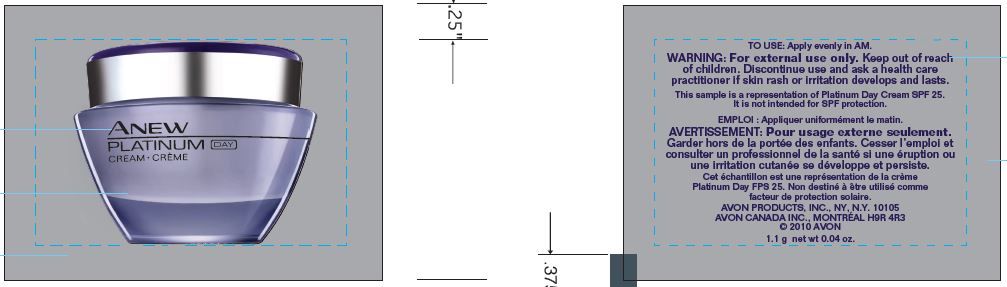
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANEW PLATINUM DAY
octinoxate, octisalate, oxybenzone, avobenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71110-0009 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 47.5 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 40 mg in 1 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 28.5 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TRISILOXANE (UNII: 9G1ZW13R0G) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) PEG-100 STEARATE (UNII: YD01N1999R) GLYCERIN (UNII: PDC6A3C0OX) THIAZOLYLALANINE (UNII: VTL1Y3S44B) MALTODEXTRIN (UNII: 7CVR7L4A2D) CAPRYLYL GLYCOL (UNII: 00YIU5438U) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) DIMETHICONE (UNII: 92RU3N3Y1O) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71110-0009-1 1.1 g in 1 PACKET; Type 0: Not a Combination Product 03/01/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/01/2016 Labeler - Avon Products, Inc. (001468693) Establishment Name Address ID/FEI Business Operations Avon Manufacturing (Guangzhou) Ltd 544863277 manufacture(71110-0009)